Abstract
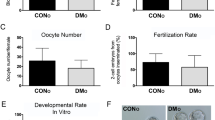
Diabetes mellitus (DM) disturbs normal functions from the level of cells to the level of organs. In this study, the authors explore the detrimental effects of type 1 diabetes on meiotic regulation depending on the duration of DM. In non—diabetes-prone BioBreeding (BBdr) control rats, most of the large follicles had germinal vesicle (GV)—intact oocytes. Conversely, a decrease of intact GV that was dependent on the duration of diabetic symptoms was observed; only 54% of the large follicles of diabetes-prone BB (BBdp) rats had GV-intact oocytes at 6 weeks after diabetes induction. Furthermore, some of the secondary follicles in BBdp rats also had germinal vesicle breakdown (GVB) oocytes. The nuclear status of the euglycemia BBdp rat was similar to those of the BBdr rat. In BBdp rats, the rate of meiotic progression to the metaphase II stage was significantly lower; however, the rate of segregated oocytes was significantly increased compared with controls during induction of in vitro maturation. The rate of segregated oocytes was not affected by the presence of the cumulus after chronic symptoms. These results indicate that chronic DM has a detrimental effect on meiotic regulation during folliculogenesis and results in a reduced number of competent oocytes. In addition, these data suggest that the follicle cells can resume supporting the meiotic regulation under euglycemia through insulin administration, independent of the duration of DM.
Similar content being viewed by others
References
Baudat F, de Massy B. SPO11: an activity that promotes DNA breaks required for meiosis. Med Sci (Paris). 2004;20:213–218.
Hashimoto N, Watanabe N, Furuta Y, et al. Pathenogenetic activation of oocytes in c-mos-deficient mice. Nature. 1994; 370:68–71.
Steffensen KR, Robertson K, Gustafsson JA, Andersen CY. Reduced fertility and inability of oocytes to resume meiosis in mice deficient of the Lxr genes. Mol Cell Endocrinol. 2006; 256:9–16.
Dumesic DA, Schramm RD, Abbott DH. Early origins of poly-cystic ovary syndrome. Reprod Fertil Dev. 2005;17:349–360.
Garris DR, Whitehead DS, Morgan CR. Effects of alloxaninduced diabetes on corpus luteum function in the pseudo-pregnant rat. Diabetes. 1984;33:611–615.
Griffin ML, South SA, Yankov VI, et al. Insulin-dependent diabetes mellitus and menstrual dysfunction. Ann Med. 1994; 26:331–340.
Holemans K, Aerts L, van Assche FA. Fetal growth restriction and consequences for the offspring in animal models. J Soc Gynecol Investig. 2003;10:392–399.
Kanter JE, Johansson F, LeBoueuf RC, Bornfeldt KE. Do glucose and lipids exert independent effects on atherosclerotic lesion initiation or progression to advanced plaques? Circ Res. 2007;100:769–781.
Halmos T. New diagnostic and classification system in diabetic syndrome. Orv Hetil. 2002;143:2533–2541.
Babichev VN, Adamskaia EI, Peryshkova TA. Analysis of hypothalamo-hypophyseal-gonadal interrelationships in female rats in experimentally induced diabetes. Probl Endokrinol Mosk. 1994;40:46–50.
Becerra JE, Khoury MJ, Cordero JF, Erickson JD. Diabetes mellitus during pregnancy and the risks due specific birth defects: a population based case-control study. Pediatrics. 1990; 85:1–9.
Garris DR, Smith C, Diani AT, Gerritsen GC. Morphometric evaluation of the hypothalamic-ovarian axis of the ketonuri, diabetic Chinese hamster: relationship to the reproductive cycle. Diabetologia. 1982;23:275–279.
Tesone M, Landenheim RG, Cheb-Terrab R, et al. Comparison between bioactive and immunoactive luteinizing hormone (LH) in ovariectomized streptozotocin-induced diabetic rats: response to LH-releasing hormone. Endocrinology. 1986;119: 2412–2416.
Powers RW, Chambers C, Larsen WJ. Diabetes-mediated decreases in ovarian superoxide dismutase activity are related to blood-follicle barrier and ovulation defects. Endocrinology. 1996;137:301–310.
Garris DR, Williams SK, West L. Morphometric evaluation of diabetes-associated ovarian atrophy in the C57/KsJ mouse: relationship to age and ovarian function. Anat Rec. 1985; 211:434–443.
Diamond MP, Lavy G, Polan ML. Progesterone production from granulose cells of individual human follicles derived from diabetic and nondiabetic subjects. Int J Fertil. 1989;34:204–208.
Edwards JL, Hughey TC, Moore AB, Cox NM. Depletion of insulin in streptozocin-induced-diabetic pigs alters estradiol, insulin-like growth factor (IGF)-1, and IGF binding proteins in cultured ovarian follicles. Biol Reprod. 1996;55:775–781.
Colton SA, Pieper GM, Downs SM. Altered meiotic regulation in oocytes from diabetic mice. Biol Reprod. 2002;67:220–231.
Chang AS, Dale AN, Moley KH. Maternal diabetes adversely affects preovulatory oocyte maturation, development, and granulose cell apoptosis. Endocrinology. 2005;146:2445–2453.
Chakir H, Lefebvre DE, Wang H, Caraher E, Scott FW. Wheat protein-induced proinflammatory T helper 1 bias in mesenteric lymph nodes of young diabetes-prone rats. Diabetologia. 2005;48:1576–1584.
Herbert M, Levasseur M, Homer H, Yallop K, Murdoch A, McDougall A. Homologue disjunction in mouse oocytes requires proteolysis of securing and cyclin B1. Nat Cell Biol. 2003;5:1023–1025.
Papin C, Rouget C, Lorca T, Castro A, Mandart E. XCdh1 is involved in progesterone-induce oocyte maturation. Dev Biol. 2004;272:66–75.
Downs SM, Cottom J, Hunzicker-Dunn M. Protein kinase C and meiotic regulation in isolated mouse oocytes. Mol Reprod Dev. 2001;58:101–115.
Downs SM, Daniel SA, Eppig JJ. Induction of maturation in cumulus cell-enclosed mouse oocytes by FSH and EGF: evidence for a positive stimulus of somatic cell origin. J Exp Zool. 1988;245:86–96.
Colton SA, Downs SM. Potential role for the sorbitol pathway in the meiotic dysfunction exhibited by oocytes from diabetic mice. J Exp Zoolog A Comp Exp Biol. 2004;301A:439–448.
Gilbert SF. The saga of the germ line. In: Gilbert SF, ed. Developmental Biology. 7th ed. Sunderland, MA: Sinauer; 2003: 613–646.
Diamond MP, Pettway ZY, Logan J, Moley K, Vaughn W, DeCherney AH. Dose response effects of glucose, insulin, and glucagons on mouse pre-embryo development. Metabolism. 1991;40:566–570.
Ackert CL, Gittens Jei, O’Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol. 2001;233:258–270.
Atef A, Francois P, Christian V, Marc-Andre S. The potential role of gap junction communication between cumulus cells and bovine oocytes during in vitro maturation. Mol Reprod Dev. 2005;71:358–367.
Fan HY, Huo LJ, Chen DY, Schatten H, Sun QY. Protein kinase C and mitogen activated protein kinase cascade in mouse cumulus cells: cross talk and effect on meiotic resumption of oocyte. Biol Reprod. 2003;70:1178–1187.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Sungshin Women’s University Research grant of 2006.
Rights and permissions
About this article
Cite this article
Kim, K., Kim, C.H., Moley, K.H. et al. Disordered Meiotic Regulation of Oocytes by Duration of Diabetes Mellitus in BBdp Rat. Reprod. Sci. 14, 467–474 (2007). https://doi.org/10.1177/1933719107306228
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719107306228