Abstract
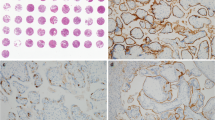
Recent studies showed that considerable amounts of glycosaminoglycans are released into maternal blood during normal pregnancy and in hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome. Maternal endothelia and the syncytiotrophoblast layer have been discussed as a possible origin of these glycocalyx components. Our study aimed to visualize the glycocalyx on the syncytiotrophoblast by electron microscopy, to analyze its structure and composition by immunohistochemistry, and to determine potential differences between healthy women and women with HELLP syndrome. For electron microscopy, a cotyledon was fixed by perfusion of the intervillous space with a 2% lanthanum–nitrate glutaraldehyde solution followed by immersion fixation in the same fixative. For immunohistochemistry, sections of 16 placentas (HELLP patients/healthy women, n = 8 each) were stained with monoclonal antibodies against the main glycocalyx constituents syndecan 1, hyaluronic acid, and heparan sulfate. Semiquantitative evaluation of staining intensity focused on the apical surface of the syncytiotrophoblast and fetal intravillous endothelia as possible localizations of a placental glycocalyx. Electron microscopy revealed a glycocalyx of approximately 250 nm, covering the syncytiotrophoblast layer. This was found to contain large amounts of syndecan 1, but neither hyaluronic acid nor heparan sulfate as major components. Intravillous fetal endothelium did not express any of the investigated glycosaminoglycans. Healthy women and patients with HELLP showed no differences concerning glycocalyx composition and thickness of the syncytiotrophoblast. The composition of the “placental” glycocalyx differs from the adult and fetal vascular glycocalyx. Obviously, the human placental syncytiotrophoblast maintains a special kind of glycocalyx at the fetomaternal interface.
Similar content being viewed by others
References
Hofmann-Kiefer KF, Kemming GI, Chappell D, et al. Serum heparan sulfate levels are elevated in endotoxemia. Eur J Med Res. 2009;14(12):526–531.
Platts SH, Linden J, Duling BR. Rapid modification of the glycocalyx caused by ischemia-reperfusion is inhibited by adenosine A2A receptor activation. Am J Physiol Heart Circ Physiol. 2003;284(6):H2360–H2367.
Gouverneur M, Berg B, Nieuwdorp M, Stroes E, Vink H. Vasculoprotective properties of the endothelial glycocalyx: effects of fluid shear stress. J Intern Med. 2006;259(4):393–400.
Chappell D, Hofmann-Kiefer K, Jacob M, et al. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol. 2009; 104(1):78–89.
Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer: implications for plateletendothelial cell adhesion. Circulation. 2000;101(13):1500–1502.
Crescimanno C, Marzioni D, Paradinas FJ, et al. Expression pattern alterations of syndecans and glypican-1 in normal and pathological trophoblast 1. J Pathol. 1999;189(4):600–608.
Lorenzi T, Turi A, Crescimanno C, et al. Syndecan expressions in the human amnion and chorionic plate. Eur J Histochem. 2010; 54(4):e42.
Matejevic D, Neudeck H, Graf R, Muller T, Dietl J. Localization of hyaluronan with a hyaluronan-specific hyaluronic acid binding protein in the placenta in pre-eclampsia. Gynecol Obstet Invest. 2001;52(4):257–259.
Chappell D, Jacob M, Paul JO, et al. The glycocalyx of the human umbilical vein endothelial cell—an impressive structure ex vivo, but not in culture. Circ Res. 2009;104(11):1013–1017.
Barker AL, Konopatskaya O, Neal CR, et al. Observation and characterisation of the glycocalyx of viable human endothelial cells using confocal laser scanning microscopy. Phys Chem Chem Phys. 2004;6(5):1006–1011.
Hofmann-Kiefer KF, Knabl J, Martinoff N, et al. Increased serum concentrations of circulating glycocalyx components in HELLP syndrome compared to healthy pregnancy: an observational study. Reprod Sci. 2013;20(3):318–325.
Nelson A, Berkestedt I, Schmidtchen A, Ljunggren L, Bodelsson M. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock. 2008;30(6):623–627.
Martin JN Jr., Rinehart BK, May WL, Magann EF, Terrone DA, Blake PG. The spectrum of severe preeclampsia: comparative analysis by HELLP (hemolysis, elevated liver enzyme levels, and low platelet count) syndrome classification. Am J Obstet Gynecol. 1999;180(6 pt 1):1373–1384.
Haram K, Svendsen E, Abildgaard U. The HELLP syndrome: clinical issues and management. A Review. BMC Pregnancy Childbirth. 2009;9:8.
Rehm M, Zahler S, Lotsch M, et al. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology. 2004;100(5):1211–1223.
Klein M, Vignaud JM, Hennequin V, et al. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2001;86(2):656–658.
Jones CJ, Fox H. Ultrastructure of the normal human placenta. Electron Microsc Rev. 1991;4(1):129–178.
Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79(3):581–589.
van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003;92(6): 592–594.
Luft JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966;25(6):1773–1783.
Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167.
Bachmaier N, Linnemann K, May K, et al. Ultrastructure of human placental tissue after 6 h of normoxic and hypoxic dual in vitro placental perfusion. Placenta. 2007;28(8–9):861–867.
Haigh M, Chawner LE, Fox H. The human placenta does not contain lipofuscin pigment. Placenta. 1984;5(5):459–464.
Eaton BM, Leach L, Firth JA. Permeability of the fetal villous microvasculature in the isolated perfused term human placenta. J Physiol. 1993;463:141–155.
Chen CP, Liu SH, Lee MY, Chen YY. Heparan sulfate proteoglycans in the basement membranes of the human placenta and decidua. Placenta. 2008;29(4):309–316.
Haimov-Kochman R, Friedmann Y, Prus D, et al. Localization of heparanase in normal and pathological human placenta. Mol Hum Reprod. 2002;8(6):566–573.
Muhlhauser J, Marzioni D, Morroni M, Vuckovic M, Crescimanno C, Castellucci M. Codistribution of basic fibroblast growth factor and heparan sulfate proteoglycan in the growth zones of the human placenta 2. Cell Tissue Res. 1996;285(1):101–107.
Jokimaa V, Inki P, Kujari H, Hirvonen O, Ekholm E, Anttila L. Expression of syndecan-1 in human placenta and decidua. Placenta. 1998;19(2–3):157–163.
Jokimaa VI, Kujari HP, Ekholm EM, Inki PL, Anttila L. Placental expression of syndecan 1 is diminished in preeclampsia Am J Obstet Gynecol. 2000;183(6):1495–1498.
Gao L, Lipowsky HH. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes 1. Microvasc Res. 2010;80(3):394–401.
Wasserman L, Abramovici A, Shlesinger H, Goldman JA, Allalouf D. Histochemical localization of acidic glycosaminoglycans in normal human placentae 1. Placenta. 1983;4(1):101–108.
Steegers EA, von DP, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–644.
Gifford RW. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22.
van Runnard Heimel PJ, Kavelaars A, Heijnen CJ, et al. HELLP syndrome is associated with an increased inflammatory response, which may be inhibited by administration of prednisolone. Hypertens Pregnancy. 2008;27(3):253–265.
Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606): 75–84.
Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(suppl 3):17–42.
Bernal A. Overview. Preterm labour: mechanisms and management. BMC Pregnancy Childbirth. 2007;7(suppl 1):S2.
Norman JE. Preterm labour. Cervical function and prematurity. Best Pract Res Clin Obstet Gynaecol. 2007;21(5):791–806.
Sankaran S, Kyle PM. Aetiology and pathogenesis of IUGR. Best Pract Res Clin Obstet Gynaecol. 2009;23(6):765–777.
Rehm M, Bruegger D, Christ F, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116(17): 1896–1906.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hofmann-Kiefer, K.F., Chappell, D., Knabl, J. et al. Placental Syncytiotrophoblast Maintains a Specific Type of Glycocalyx at the Fetomaternal Border: The Glycocalyx at the Fetomaternal Interface in Healthy Women and Patients With HELLP Syndrome. Reprod. Sci. 20, 1237–1245 (2013). https://doi.org/10.1177/1933719113483011
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719113483011