Abstract
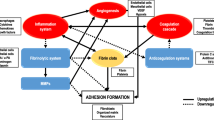
Over the past several years, there has been increasing recognition that pathogenesis of adhesion development includes significant contributions of hypoxia induced at the site of surgery, the resulting oxidative stress, and the subsequent free radical production. Mitochondrial dysfunction generated by surgically induced tissue hypoxia and inflammation can lead to the production of reactive oxygen and nitrogen species as well as antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase which when optimal have the potential to abrogate mitochondrial dysfunction and oxidative stress, preventing the cascade of events leading to the development of adhesions in injured peritoneum. There is a significant cross talk between the several processes leading to whether or not adhesions would eventually develop. Several of these processes present avenues for the development of measures that can help in abrogating adhesion formation or reformation after intraabdominal surgery.
Similar content being viewed by others
References
Agarwal A, Allamaneni SS. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod Biomed Online. 2004;9(3):338–347.
Halliwell BG, John Gutteridge, eds. Free Radicals in Biology and Medicine. Oxford: Clarendon; 1989:1–20.
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84.
Yung LM, Leung FP, Yao X, Chen ZY, Huang Y. Reactive oxygen species in vascular wall. Cardiovasc Hematol Disord Drug Targets. 2006;6(1):1–19.
Crimi E, Ignarro LJ, Napoli C. Microcirculation and oxidative stress. Free Radic Res. 2007;41(12):1364–1375.
Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertility—a clinician’s perspective. Reprod Biomed Online. 2005;ll(5):641–650.
Fissore RA, Kurokawa M, Knott J, Zhang M, Smyth J. Mechanisms underlying oocyte activation and postovulatory ageing. Reproduction. 2002;124(6):745–754.
Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM. Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic Biol Med. 2008;44(7):1295–1304.
Goud AP, Goud PT, Diamond MP, Abu-Soud HM. Nitric oxide delays oocyte aging. Biochemistry. 2005;44(34):11361–11368.
Rizzo A, Roscino M, Binetti F, Sciorsci R. Roles of reactive oxygen species in female reproduction. Reprod Domest Anim. 2012;47(2):344–352.
Clerici G, Slavescu C, Fiengo S, et al. Oxidative stress in pathological pregnancies. J Obstet Gynaecol. 2012;32(2): 124–127.
Yildirim G, Attar R, Ozkan F, Kumbak B, Ficicioglu C, Yesilda-glar N. The effects of letrozole and melatonin on surgically induced endometriosis in a rat model: a preliminary study. Fertil Steril. 2010;93(6):1787–1792.
Taddei ML, Giannoni E, Raugei G, et al. Mitochondrial oxidative stress due to complex I dysfunction promotes fibroblast activation and melanoma cell invasiveness. J Signal Transduct. 2012;2012: 684592.
Diamond MP. Surgical aspects of infertility. In: Sciarra JW, ed. Gynecology and Obstetrics. Vol 2. Philadelphia: Harper & Row; 1988:1–23.
Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study, seprafilm adhesion study group. Fertil Steril. 1996;66(6):904–910.
Diamond MP. Incidence of postsurgical adhesions. In: GS d diZerega, ed. Peritoneal Surgery. New York: Spriger-Verlag; 2000:217–220.
Awonuga AO, Fletcher NM, Saed GM, Diamond MP. Postoperative adhesion development following cesarean and open intra-abdominal gynecological operations: a review. Reprod Sci. 2011;18(12):1166–1185.
Vrijland WW JJ, van Geldorp HJ, Swank DJ, Bonjer HJ. Abdominal adhesions: intestinal obstruction, pain, and infertility. Surg Endose. 2003;17(7):1017–1022.
Marana R, Catalano GF, Muzii L. Salpingoscopy. Curr Opin Obstet Gynecol. 2003;15(4):333–336.
Barmparas G, Branco BC, Schnuriger B, Lam L, Inaba K, Demetriades D. The incidence and risk factors of post-laparotomy adhesive small bowel obstruction. J Gastrointest Surg. 2010;14(10):1619–1628.
Duffy DM, diZerega GS. Adhesion controversies: pelvic pain as a cause of adhesions, crystalloids in preventing them. J Reprod Med. 1996;41(1):19–26.
Holmdahl L, Risberg B. Adhesions: prevention and complications in general surgery. Eur J Surg. 1997;163(3):169–174.
Murdoch C, Lewis CE. Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer. 2005;117(5): 701–708.
Terada LS, Guidot DM, Leff JA, et al. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc Natl Acad Sci U S A. 1992;89(8):3362–3366.
Fridovich I. The biology of oxygen radicals. Science. 1978; 201(4359):875–880.
Yamamoto Y, Konig P, Henrich M, Dedio J, Kummer W. Hypoxia induces production of nitric oxide and reactive oxygen species in glomus cells of rat carotid body. Cell Tissue Res. 2006;325(1):3–11.
Zhu H, Bunn HF. Oxygen sensing and signaling: impact on the regulation of physiologically important genes. Respir Physiol. 1999;115(2):239–247.
McNally JS, Davis ME, Giddens DP, et al. Role of xanthine oxi-doreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285(6):H2290–H2297.
Stuehr DJ, Cho HJ, Kwon NS, Weise MF, Nathan CF. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavopro-tein. Proc Natl Acad Sci U S A. 1991;88(17):7773–7777.
Yui Y, Hattori R, Kosuga K, Eizawa H, Hiki K, Kawai C. Purification of nitric oxide synthase from rat macrophages. J Biol Chem. 1991;266(19): 12544–12547.
Stuehr DJ. Structure-function aspects in the nitric oxide synthases. Annu Rev Pharmacol Toxicol. 1997;37:339–359.
Chinje EC, Stratford IJ. Role of nitric oxide in growth of solid tumours: a balancing act. Essays Biochem. 1997;32:61–72.
MacMicking JD, Nathan C, Hom G, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81(4): 641–650.
Kettle AJ, van Dalen CJ, Winterbourn CC. Peroxynitrite and myeloperoxidase leave the same footprint in protein nitration. Redox Rep. 1997;3(5–6):257–258.
Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils, evidence for hypochlorous acid generation. J Clin Invest. 1982;70(3):598–607.
Tahboub YR, Galijasevic S, Diamond MP, Abu-Soud HM. Thio-cyanate modulates the catalytic activity of mammalian peroxidases. J Biol Chem. 2005;280(28):26129–26136.
Pullar JM, Vissers MC, Winterbourn CC. Living with a killer: the effects of hypochlorous acid on mammalian cells. IUBMB Life. 2000;50(4–5):259–266.
Pascoe GA, Fariss MW, Olafsdottir K, Reed DJ. A role of vitamin E in protection against cell injury. Maintenance of intracellular glutathione precursors and biosynthesis. Eur J Biochem. 1987;166(1):241–247.
Harrison FE, Meredith ME, Dawes SM, Saskowski JL, May JM. Low ascorbic acid and increased oxidative stress in gulo(-/-) mice during development. Brain Res. 2010;1349:143–152.
Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240.
Akaike T, Suga M, Maeda H. Free radicals in viral pathogenesis: molecular mechanisms involving superoxide and NO. Proc Soc Exp Biol Med. 1998;217(1):64–73.
Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev. 2002;54(4):619–634.
Fletcher NM, Jiang ZL, Diamond MP, Abu Soud HM, Saed GM. Hypoxia-generated superoxide induces the development of the adhesion phenotype. Free Radic Biol Med. 2008;45(4):530–536.
Szczepanska M, Kozlik J, Skrzypczak J, Mikolajczyk M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil Steril. 2003;79(6):1288–1293.
Van Langendonckt A, Casanas Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril. 2002;77(5): 861–870.
El Mouatassim S, Guerin P, Menezo Y. Mammalian oviduct and protection against free oxygen radicals: expression of genes encoding antioxidant enzymes in human and mouse. Eur J Obstet Gynecol Reprod Biol. 2000;89(1):1–6.
Saed GM, Diamond MP. Molecular characterization of postoperative adhesions: the adhesion phenotype. J Am Assoc Gynecol Laparosc. 2004;11(3):307–314.
Li YQ BJ, Nordal RA, Su ZF, Wong CS. Hypoxia in radiation-induced blood spinal cord barrier breakdown. Cancer Res Treat. 2000;61(8):3348–3354.
Reed KL, Heydrick SJ, Aarons CB, et al. A neurokinin-1 receptor antagonist that reduces intra-abdominal adhesion formation decreases oxidative stress in the peritoneum. Am J Physiol Gas-trointest Liver Physiol. 2007;293(3):G544–G551.
Heydrick SJ, Reed KL, Cohen PA, et al. Intraperitoneal administration of methylene blue attenuates oxidative stress, increases peritoneal fibrinolysis, and inhibits intraabdominal adhesion formation. J Surg Res. 2007;143(2):311–319.
Dunn RC, Buttram VC Jr. Tissue-type plasminogen activator as an adjuvant for post surgical adhesions. Prog Clin Biol Res. 1990;358:113–118.
Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146(1):56–66.
Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277(2 pt 1):C183–C201.
Sogawa K. Overview: hypoxia [in Japanese]. Tanpakushitsu KakusanKoso. 1999;44(15 suppl):2470–2471.
Saed GM, Diamond MP. Hypoxia-induced irreversible up-regulation of type I collagen and transforming growth factor-betal in human peritoneal fibroblasts. Fertil Steril. 2002;78(1): 144–147.
Alpay Z, Ozgonenel M, Savasan S, Buck S, Saed GM, Diamond MP. Altered in vitro immune response to hypoxia-treated normal peritoneal fibroblasts. Fertil Steril. 2007;87(2):426–429.
Falanga V. Wound healing. an overview. J Dermatol Surg Oncol. 1993;19(8):689–690.
Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1): 103–111.
Saed GM, Diamond MP. Differential expression of alpha smooth muscle cell actin in human fibroblasts isolated from intraperitoneal adhesions and normal peritoneal tissues. Fertil Steril. 2004; 82 suppl 3:1188–1192.
Saed GM, Abu-Soud HM, Diamond MP. Role of nitric oxide in apoptosis of human peritoneal and adhesion fibroblasts after hypoxia. Fertil Steril. 2004;82 suppl 3:1198–1205.
Shavell VI, Saed GM, Diamond MP. Review: cellular metabolism: contribution to postoperative adhesion development. Reprod Sci. 2009;16(7):627–634.
Cookson VJ, Chapman NR. NF-kappaB function in the human myometrium during pregnancy and parturition. Histol Histo-pathol. 2010;25(7):945–956.
Saed GM, Diamond MP. Apoptosis and proliferation of human peritoneal fibroblasts in response to hypoxia. Fertil Steril. 2002;78(1):137–143.
Chegini N. The role of growth factors in peritoneal healing: transforming growth factor beta (TGF-beta). Eur J Surg Suppl. 1997;(577):17–23.
Idell S, Zwieb C, Boggaram J, Holiday D, Johnson AR, Raghu G. Mechanisms of fibrin formation and lysis by human lung fibroblasts: influence of TGF-beta and TNF-alpha. Am J Physiol. 1992;263(4 pt 1):L487–L494.
Saed GM, Zhang W, Diamond MP. Molecular characterization of fibroblasts isolated from human peritoneum and adhesions. Fertil Steril. 2001;75(4):763–768.
Ivarsson ML, Holmdahl L, Falk P, Molne J, Risberg B. Characterization and fibrinolytic properties of mesothelial cells isolated from peritoneal lavage. Scand J Clin Lab Invest. 1998;58(3): 195–203.
Diamond MP, El-Hammady E, Munkarah A, Bieber EJ, Saed G. Modulation of the expression of vascular endothelial growth factor in human fibroblasts. Fertil Steril. 2005;83(2):405–409.
Saed GM, Zhang W, Chegini N, Holmdahl L, Diamond MP. Alteration of type I and III collagen expression in human peritoneal mesothelial cells in response to hypoxia and transforming growth factor-betal. Wound Repair Regen. 1999;7(6):504–510.
Saed GM, Munkarah AR, Abu-Soud HM, Diamond MP. Hypoxia upregulates cyclooxygenase-2 and prostaglandin E(2) levels in human peritoneal fibroblasts. Fertil Steril. 2005;83 suppl 1:1216–1219.
Saed GM, Diamond MP. Modulation of the expression of tissue plasminogen activator and its inhibitor by hypoxia in human peritoneal and adhesion fibroblasts. Fertil Steril. 2003;79(1): 164–168.
Lu Y, Wahl LM. Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NF-kappa B activity in lipopolysaccharide-activated human primary monocytes. J Immunol. 2005;175(8):5423–5429.
Saed GM, Zhao M, Diamond MP, Abu-Soud HM. Regulation of inducible nitric oxide synthase in post-operative adhesions. Hum Reprod. 2006;21(6):1605–1611.
Abu-Soud HM, Stuehr DJ. Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci U S A. 1993;90(22):10769–10772.
Rosen GM, Tsai P, Weaver J, et al. The role of tetrahydrobiopterin in the regulation of neuronal nitric-oxide synthase-generated superoxide. J Biol Chem. 2002;277(43):40275–40280.
Jiang ZL, Fletcher NM, Diamond MP, Abu-Soud HM, Saed GM. S-nitrosylation of caspase-3 is the mechanism by which adhesion fibroblasts manifest lower apoptosis. Wound Repair Regen. 2009;17(2):224–229.
Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radie Biol Med. 1999; 26(3–4):463–471.
Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998;13(4):998–1002.
Tarin JJ, Gomez-Piquer V, Pertusa JF, Hermenegildo C, Cano A. Association of female aging with decreased parthenogenetic activation, raised MPF, and MAPKs activities and reduced levels of glutathione S-transferases activity and thiols in mouse oocytes. Mol Reprod Dev. 2004;69(4):402–410.
Gardiner CS, Salmen JJ, Brandt CJ, Stover SK. Glutathione is present in reproductive tract secretions and improves development of mouse embryos after chemically induced glutathione depletion. Biol Reprod. 1998;59(2):431–436.
Shukla A, Rasik AM, Shankar R. Nitric oxide inhibits wounds collagen synthesis. Mol Cell Biochem. 1999;200(1–2):27–33.
Ferrini MG, Vernet D, Magee TR, et al. Antifibrotic role of inducible nitric oxide synthase. Nitric Oxide. 2002;6(3):283–294.
Jiang ZL, Zhu X, Diamond MP, Abu-Soud HM, Saed GM. Nitric oxide synthase isoforms expression in fibroblasts isolated from human normal peritoneum and adhesion tissues. Fertil Steril. 2008;90(3):769–774.
Cudd A, Fridovich I. Electrostatic interactions in the reaction mechanism of bovine erythrocyte superoxide dismutase. J Biol Chem. 1982;257(19):11443–11447.
van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272(12):7617–7625.
Hazen SL, Zhang R, Shen Z, et al. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation In vivo. Circ Res. 1999;85(10):950–958.
Jong EC, Klebanoff SJ. Eosinophil-mediated mammalian tumor cell cytotoxicity: role of the peroxidase system. J Immunol. 1980;124(4):1949–1953.
Mayeno AN, Curran AJ, Roberts RL, Foote CS. Eosinophils preferentially use bromide to generate halogenating agents. J Biol Chem. 1989;264(10):5660–5668.
Klebanoff SJ, Waltersdorph AM, Rosen H. Antimicrobial activity of myeloperoxidase. Methods Enzymol. 1984; 105: 399–403.
Stacker R, Keaney JF Jr. Role of oxidative modification in atherosclerosis. Physiol Rev. 2004;84(4):1381–1478.
Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275(48): 37524–37532.
Abu-Soud HM, Hazen SL. Nitric oxide modulates the catalytic activity of myeloperoxidase. J Biol Chem. 2000;275(8): 5425–5430.
Abu-Soud HM, Hazen SL. Interrogation of heme pocket environment of mammalian peroxidases with diatomic ligands. Bio-chemistry. 2001;40(36):10747–10755.
Galijasevic S, Saed GM, Diamond MP, Abu-Soud HM. Myeloperoxidase up-regulates the catalytic activity of inducible nitric oxide synthase by preventing nitric oxide feedback inhibition. Proc Natl Acad Sci U S A. 2003;100(25):14766–14771.
Abu-Soud HM, Khassawneh MY, Sohn JT, Murray P, Haxhiu MA, Hazen SL. Peroxidases inhibit nitric oxide (NO) dependent bronchodilation: development of a model describing NO-peroxidase interactions. Biochemistry. 2001;40(39):11866–11875.
Kettle AJ, Winterbourn CC. Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep. 1997;3(3):3–15.
Abu-Soud HM, Raushel FM, Hazen SL. A novel multistep mechanism for oxygen binding to ferrous hemoproteins: rapid kinetic analysis of ferrous-dioxy myeloperoxidase (compound III) formation. Biochemistry. 2004;43(36):11589–11595.
Shao B, Oda MN, Vaisar T, Oram JF, Heinecke JW. Pathways for oxidation of high-density lipoprotein in human cardiovascular disease. Curr Opin Mol Ther. 2006;8(3):198–205.
Xu W, Zheng S, Dweik RA, Erzurum SC. Role of epithelial nitric oxide in airway viral infection. Free Radic Biol Med. 2006;41(1):19–28.
Westman E, Lundberg K, Erlandsson Harris H. Arthritogenicity of collagen type II is increased by chlorination. Clin Exp Immunol. 2006;145(2):339–345.
Midwinter RG, Vissers MC, Winterbourn CC. Hypochlorous acid stimulation of the mitogen-activated protein kinase pathway enhances cell survival. Arch Biochem Biophys. 2001;394(1): 13–20.
McCord JM, Fridovich I. Superoxide dismutase. an enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969; 244(22):6049–6055.
Schallreuter KU, Moore J, Wood JM, et al. In vivo and in vitro evidence for hydrogen peroxide (H202) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J Investig Dermatol Symp Proc. 1999;4(1):91–96.
Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59(3):527–605.
Halliwell B.Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: measurement, mechanism and the effects of nutrition. Mutat Res. 1999;443(1–2):37–52.
Balasubramanian B, Pogozelski WK, Tullius TD. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc Natl Acad Sci U S A. 1998;95(17):9738–9743.
Diamond MP, El-Hammady E, Wang R, Kruger M, Saed G. Regulation of expression of tissue plasminogen activator and plasminogen activator inhibitor-1 by dichloroacetic acid in human fibroblasts from normal peritoneum and adhesions. Am J Obstet Gynecol. 2004;190(4):926–934.
Gomel V. The impact of microsurgery in gynecology. Clin Obstet Gynecol. 1980;23(4):1301–1310.
Winston RM. Microsurgery of the fallopian tube: from fantasy to reality. Fertil Steril. 1980;34(6):521–530.
Awonuga AO, Saed GM, Diamond MP. Laparoscopy in gynecologic surgery: adhesion development, prevention, and use of adjunctive therapies. Clin Obstet Gynecol. 2009;52(3):412–422.
Lundorff P, Hahlin M, Kallfelt B, Thorburn J, Lindblom B. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55(5):911–915.
Milingos S, Kallipolitis G, Loutradis D, et al. Adhesions: laparoscopic surgery versus laparotomy. Ann N Y Acad Sci. 2000;900: 272–285.
Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. operative laparoscopy study group. Fertil Steril. 1991 ;55(4): 700–704.
Ott DE. Desertification of the peritoneum by thin-film evaporation during laparoscopy. JSLS. 2003;7(3):189–195.
Yesildaglar N, Ordonez JL, Laermans I, Koninckx PR. The mouse as a model to study adhesion formation following endoscopic surgery: a preliminary report. Hum Reprod. 1999;14(1): 55–59.
Michelson AM, Puget K. [Medical aspects of superoxide dismu-tases]. C R Seances Soc Biol Fil. 1979;173(2):380–393.
Lefaix JL, Delanian S, Leplat JJ, et al. Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD and Mn-SOD: an experimental study. Int J Radiat Oncol Biol Phys. 1996;35(2): 305–312.
Martin M, Lefaix J, Delanian S. TGF-betal and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47(2):277–290.
Batinic-Haberle I, Spasojevic I, Stevens RD, et al. New PEG-ylated Mn(III) porphyrins approaching catalytic activity of SOD enzyme. Dalton Trans. 2006;(4):617–624.
Ferrer-Sueta G, Hannibal L, Batinic-Haberle I, Radi R. Reduction of manganese porphyrins by flavoenzymes and submito-chondrial particles: a catalytic cycle for the reduction of peroxynitrite. Free Radic Biol Med. 2006;41(3):503–512.
Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, et al. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002; 33(6):857–863.
Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95.
Rier SE, Martin DC, Bowman RE, Dmowski WP, Becker JL. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fun-dam Appl Toxicol. 1993;21(4):433–441.
Victory R, Saed GM, Diamond MP. Antiadhesion effects of doc-osahexaenoic acid on normal human peritoneal and adhesion fibroblasts. Fertil Steril. 2007;88(6):1657–1662.
Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993;62:797–821.
Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011; 51(5):1000–1013.
Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann N Y Acad Sci. 1992;669:7–20.
Fletcher NM, Awonuga AO, Saed MG, Abu-Soud HM, Diamond MP, Saed GM. Lycopene, a powerful antioxidant, significantly reduces the development of the adhesion phenotype. Syst Biol Reprod Med. 2014;60(1):14–20.
Pennathur S, Maitra D, Byun J, et al. Potent antioxidative activity of lycopene: A potential role in scavenging hypochlorous acid. Free Radic Biol Med. 2010;49(2):205–213.
Reed KL, Stucchi AF, Leeman SE, Becker JM. Inhibitory effects of a neurokinin-1 receptor antagonist on postoperative peritoneal adhesion formation. Ann N Y Acad Sci. 2008;1144:116–126.
Kluger Y, Weinbroum A, Ben-Avraham R, Galili Y, Klausner J, Rabau M. Reduction in formation of peritoneal adhesions by methylene blue in rats: a dose response study. Eur J Surg. 2000;166(7):568–571.
Gul A, Kotan C, Dilek I, Gul T, Tas A, Berktas M. Effects of methylene blue, indigo carmine solution and autologous erythrocyte suspension on formation of adhesions after injection into rats. J Reprod Fertil. 2000;120(2):225–229.
Mendes JB, Campos PP, Rocha MA, Andrade SP. Cilostazol and pentoxifylline decrease angiogenesis, inflammation, and fibrosis in sponge-induced intraperitoneal adhesion in mice. Life Sci. 2009;84(15–16):537–543.
Chegini N, Rong H, Bennett B, Stone IK. Peritoneal fluid cytokine and eicosanoid levels and their relation to the incidence of peritoneal adhesion. J Soc Gynecol Investig. 1999; 6(3):153–157.
Saed GM, Jiang Z, Fletcher NM, Diamond MP. Modulation of the BCL-2/BAX ratio by interferon-gamma and hypoxia in human peritoneal and adhesion fibroblasts. Fertil Steril. 2008; 90(5): 1925–1930.
Saed GM, Diamond MP. Effects of interferon-gamma reverse hypoxia-stimulated extracellular matrix expression in human peritoneal and adhesion fibroblasts. Fertil Steril. 2006;85 suppl 1:1300–1305.
Yamanaka O, Saika S, Okada Y, Ooshima A, Ohnishi Y. Effects of interferon-gamma on human subconjunctival fibroblasts in the presence of TGFbeta1: reversal of TGFbeta-stimulated collagen production. Graefes Arch Clin Exp Ophthalmol. 2003; 241(2):116–124.
Galijasevic S, Abdulhamid I, Abu-Soud HM. Melatonin is a potent inhibitor for myeloperoxidase. Biochemistry. 2008; 47(8):2668–2677.
Proteasa G, Tahboub YR, Galijasevic S, Raushel FM, Abu-Soud HM. Kinetic evidence supports the existence of two halide binding sites that have a distinct impact on the heme iron microenvir-onment in myeloperoxidase. Biochemistry. 2007;46(2):398–405.
Saed GM, Al-Hendy A, Salama SA, Diamond MP. Adenovirus-mediated expression of cyclooxygenase-2 antisense reverse abnormal genetic profile of human adhesion fibroblasts. Fertil Steril. 2008;89(5 suppl):1455–1460.
Ahmad G, Duffy JM, Farquhar C, et al. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2008;(2):CD000475.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Awonuga, A.O., Belotte, J., Abuanzeh, S. et al. Advances in the Pathogenesis of Adhesion Development: The Role of Oxidative Stress. Reprod. Sci. 21, 823–836 (2014). https://doi.org/10.1177/1933719114522550
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719114522550