Abstract
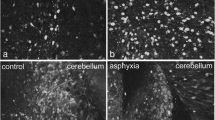
We have determined the change in immunoreactivity (IR) for microtubule-associated protein 2 (MAP-2) and synaptophysin (SYN) as markers for dendritic and presynaptic nerve development, respectively, in the ovine fetal brain with advancing gestation and in response to intermittent umbilical cord occlusion (UCO), which might then contribute to adverse neurodevelopment. Fetal sheep (control and experimental groups preterm at 111–115 and near term at 132–138 days of gestation; term = 145 days) were studied over 4 days with UCOs performed by inflation of an occluder cuff for 90 seconds every 30 minutes for 3 to 5 hours each day. Animals were then euthanized and fetal brains assessed for IR of MAP-2 and SYN. In control animals, the IR of SYN increased in the gray matter with advancing gestation consistent with a developmental increase in presynaptic vesicles and/or nerve terminals as expected; however, the IR of MAP-2 decreased in all brain regions studied, suggesting concurrent refinement in dendritic branching and spine development. Intermittent UCO as studied with marked but limited hypoxemia resulted in a decrease in IR of SYN for the brain regions of the preterm animals when protein turnover is higher and indicates decreased presynaptic vesicle formation; whereas, MAP-2 IR was selectively increased in the hippocampus CA1 and thalamus of the near-term animals, consistent with reactive dendritic change and heightened vulnerability for neuronal injury. As such, intermittent cord compressions in the ovine fetus can impact protein markers for dendritic and presynaptic nerve development depending on their timing, which might then lead to alterations in synapse formation and neuronal circuitry.
Similar content being viewed by others
References
Whitford KL, Dijkhuizen P, Polleux F, Ghosh A. Molecular control of cortical dendrite development. Annu Rev Neurosci. 2002;25:127–149.
Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508.
Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7(4):327–332.
Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol. 2010;2(4):a001842.
Vanderhaeghen P, Chen HJ. Guidance molecules in axon pruning and cell death. Cold Spring Harb Perspect Biol. 2010;2(6): a001859.
Coffey ET, Akerman KEO, Courtney MJ. Brain derived neurotrophic factor induces a rapid upregulation of synaptophysin and tau proteins via the neurotrophin receptor TrkB in rat cerebellar granule cells. Neurosci Lett. 1997;227(3):177–180.
Tang SJ, Schuman EM. Protein synthesis in the dendrite. Philos Trans R SocLond B Biol Sci. 2002;357(1420):521–529.
Burkhalter J, Fiumelli H, Allaman I, CHatton JY, Martin JL. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J Neurosci. 2003;23(23): 8212–8220.
Penn AA, Shatz CJ. Brain waves and brain wiring: the role of endogenous and sensory-drive neural activity in development. Pediatr Res. 1999;45(4 pt 1):447–458.
McIntosh GH, Baghurst KI, Potter BJ, Hetzel BS. Foetal brain development in the sheep. Neuropathol Appl Neurobiol. 1979; 5(2):103–114.
Bourgeois JP. Synaptogenesis, heterchrony and epigenesist in the mammalian neocortex. Acta Paediatr Suppl. 1997;422:27–33.
Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2): 167–178.
Low J, Handley Derry MH, Burke SO, et al. Association of intrauterine fetal growth retardation and learning deficits at age 9 to 11 years. Am J Obstet Gynecol. 1992;167(6):1499–1505.
Berg AT. Indices of fetal growth-retardation, perinatal hypoxiarelated factors and childhood neurological morbidity. Early Hum Dev. 1989;19(4):271–283.
Dalman C, Jones PB, Murray RM. Signs of asphyxia at birth and risk of schizophrenia. Population-based case-control study. Br J Psychiatry. 2001;179:403–408.
Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159(7):1080–1092.
Hultman CM, Sparén P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology. 2002;13(4):417–423.
Anyaegbunam A, Brustman L, Divon M, Langer O. The significance of antepartum variable decelerations. Am J Obstet Gynecol. 1986;155(4):707–710.
Hoskins IA, Frieden FJ, Young BK. Variable decelerations in reactive nonstress tests with decreased amniotic fluid index predict fetal compromise. Am J Obstet Gynecol. 1991;165(4 pt 1):1094–1098.
Osak R, Webster K, Bocking A, Campbell KJ, Richardson B. Nuchal cord evidence at birth impacts on fetal size relative to that of the placenta. Early Hum Dev. 1997;49(3):193–202.
Clapp JF, Lopez B, Simonean S. Nuchal cord and neurodevelopmental performance at 1 year. J Soc Gynecol Investig. 1999;6(5): 268–272.
Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179(2):507–551.
Rocha E, Hammond R, Richardson B. Necrotic cell injury in the preterm and near-term ovine fetal brain after intermittent umbilical cord occlusion. Am J Obstet Gynecol. 2004;191(2):488–496.
Falkowski A, Hammon R, Han V, Richardson B. Apoptosis in the preterm and near-term ovine fetal brain and the effect of intermittent umbilical cord occlusion. Brain Res Dev Brain Res. 2002; 136(2):165–173.
Kawagoe Y, Green L, White S, Richardson B. Intermittent umbilical cord occlusion in the ovine fetus near term: effects of behavioral state activity. Am J Obstet Gynecol. 1999;181(6): 1520–1529.
Nishigori H, Mazzuca DM, Nygard KL, Han VK, Richardson BS. BDNF and TrkB in the preterm and near-term ovine fetal brain and the effect of intermittent umbilical cord occlusion. Reprod Sci. 2008;15(9):895–905.
Rocha E, Totten S, Hammond R, Han V, Richardson B. Structural proteins during brain development in the preterm and near-term ovine fetus and the effect of intermittent umbilical cord occlusion. Am J Obstet Gynecol. 2004;191(2):497–506.
Kaneko M, White S, Homan J, Richardson B. Cerebral blood flow and metabolism in relation to electrocortical activity with severe umbilical cord occlusion in the near-term ovine fetus. Am J Obstet Gynecol 2003;188(4):961–972.
Tucker RP. The roles of microtubule-associated proteins in brain morphogenesis: a review. Brain Res Brain Res Rev. 1990;15(2): 101–120.
Johnson GV, Jope RS. The role of microtubule-associated protein 2 (MAP-2) in neuronal growth, plasticity, and degeneration. J Neurosci Res. 1992;33(4):505–512.
Eastwood SL, Burnet PW, McDonald B, Clinton J, Harrison PJ. Synaptophysin gene expression in human brain: a quantitative in situ hybridization and immunocytochemical study. Neuroscience. 1994;59(4):881–892.
Tarsa L, Goda Y. Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99(2):1012–1016.
Schwab M, Antonow-Schlorke I, Ku¨hn B, et al. Effect of antenatal betamethasone treatment on microtubule-associated proteins MAP1β and MAP2 in fetal sheep. J Physiol. 2001;530(pt 3): 497–506.
Morest DK. The growth of dendrites in the mammalian brain. Z Anat Entwicklungsgesch. 1969;128(4):290–317.
Ota A, Ikeda T, Ikenoue T, Toshimori K. Sequence of neuronal responses assessed by immunohistochemistry in the newborn rat brain after hypoxia-ischemia. Am J Obstet Gynecol 1997; 177(3):519–526.
Lingwood BE, Healy GN, Sullivan SM, Pow DV, Colditz PB. MAP2 provides reliable early assessment of neural injury in the newborn piglet model of birth asphyxia. J Neurosci Methods. 2008;171(1):140–146.
Rees S, Breen S, Loeliger M, McCrabb G, Harding R. Hypoxemia near mid-gestation has long-term effects on fetal brain development. J Neuropathol Exp Neurol. 1999;58(9):932–945.
Rees S, Breen S, Cock M, Mallard C, Stringer M, Harding R. Fetal brain injury following prolonged hypoxemia and placenta insufficiency: a review. Comp Biochem Physiol A MolIntegr Physiol. 1998;119(3):653–660.
Dieni S, Rees S. Dendritic morphology is altered in hippocampal neurons following prenatal compromise. J Neurobiol. 2003;55(1): 41–52.
Park JS, Bateman MC, Goldberg MP. Rapid alterations in dendrite morphology during sublethal hypoxia or glutamate receptor activation. Neurobiol Dis. 1996;3(3):215–227.
Drury PP, Bennet L, Booth LC, Davidson JO, Wassink G, Gunn AJ. Maturation of the mitochondrial redox response to profound asphyxia in fetal sheep. PLoS One. 2012;7(6):e39273.
Calhoun ME, Jucker M, Martin LJ, Thinakaran G, Price DL, Mouton PR. Comparative evaluation of synaptophysin-based methods for quantification of synapses. J Neurocytol. 1996; 25(12):821–828.
Cox WL, Daffos F, Forestier F, et al. Physiology and management of intrauterine growth retardation: a biologic approach with fetal blood sampling. Am J Obstet Gynecol. 1988;159(1):36–41.
West AE, Greenberg ME. Neuronal activity-regulated gene transcription in synapse development and cognitive function. Cold Spring Harb Perspect Biol. 2011;3(6).
Stamou M, Streifel KM, Goines PE, Lein PJ. Neuronal connectivity as a convergent target of gene × environment interactions that confer risk for autism spectrum disorders. Neurotoxicol Teratol. 2013;36:3–16.
Rees S, Harding R, Walker D. An adverse intrauterine environment: implications for injury and altered development of the brain. Int J Dev Neurosci. 2008;26(1):3–11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Czikk, M.J., Totten, S., Hammond, R. et al. Microtubule-Associated Protein 2 and Synaptophysin in the Preterm and Near-Term Ovine Fetal Brain and the Effect of Intermittent Umbilical Cord Occlusion. Reprod. Sci. 22, 367–376 (2015). https://doi.org/10.1177/1933719114529371
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719114529371