Abstract
Objective
Epithelial-mesenchymal transition (EMT) is essential for embryogenesis, fibrosis, and tumor metastasis. Aberrant EMT phenomenon has been reported in endometriotic tissues of patients with endometriosis (EM). In this study, we further investigated the molecular mechanism of which lipoxin A4 (LXA4) suppresses estrogen (E2)-induced EMT in EM.
Study Design
The EMT markers were analyzed by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot in eutopic endometrial epithelial cells (EECs) or investigated by immunohistochemistry and qRT-PCR in endometriotic lesion of EM mice. The invasion and migration under different treatments were assessed bytranswell assays with or without Matrigel. The messenger RNA (mRNA) and activities of matrix metalloproteinase 2 (MMP-2) and MMP-9 were determined by qRT-PCR and gelatin zymography, respectively. Luciferase reporter assay was used to measure the activity of zinc finger E-box binding homeobox I (ZEBI) promoter. The level of E2 in endometriotic tissues was assessed by enzyme-linked immunosorbent assay.
Results
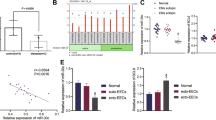
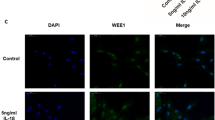
In eutopic EECs, stimulatory effects of E2 on EMT progress, migration, and invasion were all diminished by LXA4. Lipoxin A4 reduced E2-induced ZEBI promoter activity. Lipoxin A4 also attenuated the phosphorylation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase induced by E2. Co-incubation with Boc-2 rather than DMF antagonized the influence of LXA4. Animal experiments showed that LXA4 inhibited the EMT progress, MMP expression, and proteinase activities of endometriotic lesion in an LXA4 receptor (ALXR) manner, which suppressed the progression of EM. ZEBI mRNA expression was upregulated and well correlated with E2 level in human endometrium.
Conclusion
Lipoxin A4 suppresses E2-induced EMT via ALXR-dependent manner in eutopic EECs, which reveals a novel biological effect of LXA4 in EM.
Similar content being viewed by others
References
Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799.
Giudice LC, Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398.
Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–275.
Vercellini P, Crosignani P, Somigliana E, Vigano P, Frattaruolo MP, Fedele L. ‘Waiting for Godot’: a commonsense approach to the medical treatment of endometriosis. Hum Reprod. 2011;26(1):3–13.
Garcia-Velasco JA, Quea G. Medical treatment of endometriosis. Minerva Ginecol. 2005;57(3):249–255.
Platteeuw L, D’Hooghe T. Novel agents for the medical treatment of endometriosis. Curr Opin Obstet Gynecol. 2014;26(4):243–252.
Vanhie A, Tomassetti C, Peeraer K, Meuleman C, D’Hooghe T. Challenges in the development of novel therapeutic strategies for treatment of endometriosis. Expert Opin Ther Targets. 2016; 20(5):593–600.
Chen YJ, Li HY, Huang CH, et al. Oestrogen-induced epithelial-mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J Pathol. 2010;222(3):261–270.
Yoshida K, Yoshihara K, Adachi S, et al. Possible involvement of the E-cadherin gene in genetic susceptibility to endometriosis. Hum Reprod. 2012;27(6):1685–1689.
Oh SJ, Shin JH, Kim TH, et al. beta-Catenin activation contributes to the pathogenesis of adenomyosis through epithelial-mesenchymal transition. J Pathol. 2013;231(2):210–222.
Liao CJ, Li PT, Lee YC, Li SH, Chu ST. Lipocalin 2 induces the epithelial-mesenchymal transition in stressed endometrial epithelial cells: possible correlation with endometriosis development in a mouse model. Reproduction. 2014;147(2):179–187.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196.
Park SH, Cheung LW, Wong AS, Leung PC. Estrogen regulates Snail and Slug in the down-regulation of E-cadherin and induces metastatic potential of ovarian cancer cells through estrogen receptor alpha. Mol Endocrinol. 2008;22(9):2085–2098.
Maderna P, Godson C. Lipoxins: resolutionary road. Br J Pharmacol. 2009;158(4):947–959.
Serhan CN, Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101.
Li G, Wu P, Xu Y, et al. The effect of lipoxin A4 on the interaction between macrophage and osteoblast: possible role in the treatment of aseptic loosening. BMC Musculoskelet Dis. 2009;10(1):57–58.
Chinthamani S, Odusanwo O, Mondal N, Nelson J, Neelamegham S, Baker OJ. Lipoxin A4 inhibits immune cell binding to salivary epithelium and vascular endothelium. Am J Physiol Cell Physiol. 2012;302(7):968–978.
Hao H, Xu F, Hao J, et al. Lipoxin A4 suppresses lipopolysaccharide-induced hela cell proliferation and migration via NF-KB pathway. Inflammation. 2015;38(1):400–408.
Wu RF, Zhou WD, Chen S, et al. Lipoxin A4 suppresses the development of endometriosis in an ALX receptor-dependent manner via the p38 MAPK pathway. Br J Pharmacol. 2014; 171(21):4927–4940.
Serhan CN, Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr Opin Pharmacol. 2013;13(4):632–640.
Corminboeuf O, Leroy X. FPR2/ALXR agonists and the resolution of inflammation. J Med Chem. 2015;58(2):537–559.
Schaldach CM, Riby J, Bjeldanes LF. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry. 1999;38(23):7594–7600.
Machado FS, Johndrow JE, Esper L, et al. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med. 2006;12(3):330–334.
Russell R, Gori I, Pellegrini C, Kumar R, Achtari C, Canny GO. Lipoxin A4 is a novel estrogen receptor modulator. FASEB J. 2011;25(12):4326–4337.
Chen S, Wu RF, Su L, Zhou WD, Zhu MB, Chen QH. Lipoxin A4 regulates expression of the estrogen receptor and inhibits 17P-estradiol induced p38 mitogen-activated protein kinase phosphorylation in human endometriotic stromal cells. Fertil Steril. 2014;102(1):264–271.
Chen QH, Zhou WD, Pu DM, Huang QS, Li T, Chen QX. 15-Epilipoxin A(4) inhibits the progression of endometriosis in a murine model. Fertil Steril. 2010;93(5):1440–1447.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–1108.
Ran J, Lin DL, Wu RF, et al. ZEB1 promotes epithelial-mesenchymal transition in cervical cancer metastasis. Fertil Steril. 2015;103(6):1606–1614.
Robboy SJ, Bean SM. Pathogenesis of endometriosis. Reprod Biomed Online. 2010;21(1):4–5.
Shaco-Levy R, Sharabi S, Benharroch D, Piura B, Sion-Vardy N. Matrix metalloproteinases 2 and 9, E-cadherin, and beta-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):226–232.
Weigel MT, Kramer J, Schem C, et al. Differential expression of MMP-2, MMP-9 and PCNA in endometriosis and endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol. 2012;160(1):74–78.
Xin L, Hou Q, Xiong QI, Ding X. Association between matrix metalloproteinase-2 and matrix metalloproteinase-9 polymorphisms and endometriosis: a systematic review and meta-analysis. Biomed Rep. 2015;3(4):559–565.
Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis. 2008;25(6):593–600.
Wang D, Liu Y, Han J, et al. Puerarin suppresses invasion and vascularization of endometriosis tissue stimulated by 17P-estradiol. PIoS One. 2011;6(9):e25011.
Han SJ, Hawkins SM, Begum K, et al. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat Med. 2012;18(7):1102–1111.
Zhang Q, Duan J, Liu X, Guo SW. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdif-ferentiation. Mol Cell Endocrinol. 2016;428:1–16.
Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, et al. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol. 2009;23(2):265–275.
Bartley J, Julicher A, Hotz B, Mechsner S, Hotz H. Epithelial to mesenchymal transition (EMT) seems to be regulated differently in endometriosis and the endometrium. Arch Gynecol Obstet. 2014;3(5):643–650.
Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912.
Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12(1):1–13.
Ryan MB, Der CJ, Wang-Gillam A, Cox AD. Targeting -mutant cancers: is ERK the key? Trends Cancer. 2015;1(3):183–198.
Burow M. Inhibition of p38 mitogen-activated protein kinase alters microRNA expression and reverses epithelial-to-mesenchymal transition. Int J Oncol. 2013;42(4):1139–1150.
Ma B, Wells A. The mitogen-activated protein (MAP) kinases p38 and extracellular signal-regulated kinase (ERK) are involved in hepatocyte-mediated phenotypic switching in prostate cancer cells. J Biol Chem. 2014;289(16):11153–11161.
Seval Y. Estrogen-mediated regulation of p38 mitogen-activated protein kinase in human endometrium. J Clin Endocrinol Metab. 2006;91(6):2349–2357.
Matsuzaki S, Darcha C. Co-operation between the AKT and ERK signaling pathways may support growth of deep endometriosis in a fibrotic microenvironment in vitro. Hum Reprod. 2015;30(7):1606–1616.
Cheng W, Chen L, Yang S, et al. Puerarin suppresses proliferation of endometriotic stromal cells partly via the MAPK signaling pathway induced by 17P-estradiol-BSA. PLoS One. 2012;7(9):e45529.
McKinnon BD, Kocbek V, Nirgianakis K, Bersinger NA, Mueller MD. Kinase signalling pathways in endometriosis: potential targets for non-hormonal therapeutics. Hum Reprod Update. 2016;22(3). pii: dmv060.
Zhou WD, Yang HM, Wang Q, et al. SB203580, a p38 mitogen-activated protein kinase inhibitor, suppresses the development of endometriosis by down-regulating proinflammatory cytokines and proteolytic factors in a mouse model. Hum Reprod. 2010; 25(12):3110–3116.
Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127(3):299–311.
Igarashi T, Osuga U, Tsutsumi O, et al. Expression of Ah receptor and dioxin-related genes in human uterine endometrium in women with or without endometriosis. Endocr J. 1999;46(6):765–772.
Streuli I, de Ziegler D, Borghese B, et al. New treatment strategies and emerging drugs in endometriosis. Expert Opin Emerg Drugs. 2012;17(1):83–104.
Xu Z, Zhao F, Lin F, Chen J, Huang Y. Lipoxin A4 inhibits the development of endometriosis in mice: the role of anti-inflammation and anti-angiogenesis. Am J Reprod Immunol. 2012;67(6):491–497.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, RF., Huang, ZX., Ran, J. et al. Lipoxin A4 Suppresses Estrogen-Induced Epithelial-Mesenchymal Transition via ALXR-Dependent Manner in Endometriosis. Reprod. Sci. 25, 566–578 (2018). https://doi.org/10.1177/1933719117718271
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719117718271