Abstract
Infectious agents are a significant risk factor for preterm birth (PTB); however, the simple presence of bacteria is not sufficient to induce PTB in most women. Human and animal data suggest that environmental toxicant exposures may act in concert with other risk factors to promote PTB. Supporting this “second hit” hypothesis, we previously demonstrated exposure of fetal mice (Fl animals) to the environmental endocrine disruptor 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) leads to an increased risk of spontaneous and infection-mediated PTB in adult animals. Surprisingly, adult FImaes also confer an enhanced risk of PTB to their control partners. Herein, we used a recently established model of ascending group B Streptococcus (GBS) infection to explore the impact of a maternal versus paternal developmental TCDD exposure on infection-mediated PTB in adulthood. Group B Streptococcus is an important contributor to PTB in women and can have serious adverse effects on their infants. Our studies revealed that although gestation length was reduced in control mating pairs exposed to low-dose GBS, dams were able to clear the infection and bacterial transmission to pups was minimal. In contrast, exposure of pregnant Flfemales to the same GBS inoculum resulted in 100% maternal and fetal mortality. Maternal health and gestation length were not impacted in control females mated to FImales and exposed to GBS; however, neonatal survival was reduced compared to controls. Our data revealed a sex-dependent impact of parental TCDD exposure on placental expression of Toll-like receptor 2 and glycogen production, which may be responsible for the differential impact on fetal and maternal outcomes in response to GBS infection.
Similar content being viewed by others
References
Wilson MP. In the arc of history: AIHA and the movement to reform the Toxic Substances Control Act. J Occup Environ Hyg. 2012;9(5):D87–D94.
Vinceti M, Malagoli C, Teggi S, et al. Adverse pregnancy out-comes in a population exposed to the emissions of a municipal waste incinerator. Sci Total Environ. 2008;407(1):116–121.
Wang A, Padula A, Sirota M, Woodruff TJ. Environmental influences on reproductive health: the importance of chemical exposures. Fertil steril. 2016;106(4):905–929.
Mallozzi M, Bordi G, Garo C, Caserta D. The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: a review on the major concerns. Birth Defects Res C Embryo Today. 2016;108(3):224–242.
Schug TT, Johnson AF, Birnbaum LS, et al. Minireview: endocrine disrupters: past lessons and future directions. Mol endocrinol. 2016;30(8):833–847.
Boule LA, Winans B, Lawrence BP. Effects of developmental activation of the AhR on CD4+ T-cell responses to influenza virus infection in adult mice. Environ Health Perspect. 2014; 122(11):1201–1208.
Boule LA, Burke CG, Fenton BM, Thevenet-Morrison K, Jusko TA, Lawrence BP. Developmental activation of the AHR increases effector CD4+ T cells and exacerbates symptoms in autoimmune disease-prone Gnaq+/-mice. Toxicol Sci. 2015;148(2):555–566.
Naville D, Pinteur C, Vega N, et al. Low-dose food contaminants trigger sex-specific, hepatic metabolic changes in the progeny of obese mice. FASEB J. 2013;27(9):3860–3870.
Sobolewski M, Conrad K, Allen JL, et al. Sex-specific enhanced behavioral toxicity induced by maternal exposure to a mixture of low dose endocrine-disrupting chemicals. Neurotoxicology. 2014; 45:121–130.
Vanden Heuvel JP, Lucier G. Environmental toxicology of poly-chlorinated dibenzo-p-dioxins and polychlorinated dibenzofur-ans. Environ Health Perspect. 1993;100:189-200.
Bruner-Tran KL, Ding T, Yeoman KB, Archibong A, Arosh JA, Osteen KG. Developmental exposure of mice to dioxin promotes transgenerational testicular inflammation and an increased risk of preterm birth in unexposed mating partners. PLoS One. 2014; 9(8):e105084.
Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol. 2011;31 (3): 344–350.
Ding T, McConaha M, Boyd KL, Osteen KG, Bruner-Tran KL. Developmental dioxin exposure of either parent is associated with an increased risk of preterm birth in adult mice. Reprod Toxicol. 2011;31(3):351–358.
Bruner-Tran KL, Gnecco J, Ding T, Glore DR, Pensabene V, Osteen KG. Exposure to the environmental endocrine disruptor TCDD and human reproductive dysfunction: translating lessons from murine models. Reprod Toxicol. 2017;68:59-71.
Vornhagen J, Adams Waldorf KM, Rajagopal L. Perinatal group B streptococcal infections: virulence factors, immunity, and prevention strategies. Trends Microbiol. 2017;25(11):919–931.
Hansen SM, Uldbjerg N, Kilian M, Sorensen UB. Dynamics of Streptococcus agalactiae colonization in women during and after pregnancy and in their infants. J Clin Microbiol. 2004;42(1):83–89.
Kwatra G, Adrian PV, Shiri T, Buchmann EJ, Cutland CL, Madhi SA. Serotype-specific acquisition and loss of group B Streptococcus recto-vaginal colonization in late pregnancy. PLoS One. 2014; 9(6):e98778.
Namavar Jahromi B, Poorarian S, Poorbarfehee S. The prevalence and adverse effects of group B streptococcal colonization during pregnancy. Arch Iran Med. 2008;11(6):654–657.
Benitz WE, Gould JB, Druzin ML. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics. 1999;103(6):e77.
McDonald H, Vigneswaran R, O’Loughlin JA. Group B strepto-coccal colonization and preterm labour. Aust NZ J Obstet Gynaecol. 1989;29(3):291–293.
Nomura ML, Passini Junior R, Oliveira UM, Calil R. Group B Streptococcus maternal and neonatal colonization in preterm rupture of membranes and preterm labor [in Portuguese]. Rev Bras Ginecol Obstet. 2009;31(8):397–403.
McConaha ME, Ding T, Lucas JA, Arosh JA, Osteen KG, Bruner-Tran KL. Preconception omega-3 fatty acid supplementation of adult male mice with a history of developmental 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure prevents preterm birth in unexposed female partners. Reproduction. 2011;142(2):235–241.
Barton SC, Adams CA, Norris ML, Surani MA. Development of gynogenetic and parthenogenetic inner cell mass and trophectoderm tissues in reconstituted blastocysts in the mouse. J Embryol Exp Morphol. 1985;90:267-285.
Wang X, Miller DC, Harman R, Antczak DF, Clark AG. Paternally expressed genes predominate in the placenta. Proc Natl Acad Sci USA. 2013;110:10705–10710.
Binder NK, Beard SA, Kaitu’u-Lino TJ, Tong S, Hannan NJ, Gardner DK. Paternal obesity in a rodent model affects placental gene expression in a sex-specific manner. Reproduction. 2015; 149(5):435–444.
McGrath J, Solter D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell. 1984; 37(1):179–183.
Mitchell M, Strick R, Strissel PL, et al. Gene expression and epigenetic aberrations in Fl-placentas fathered by obese males. Mol Reprod Dev. 2017;84(4):316–328.
John R, Hemberger M. A placenta for life. Reprod Biomed Online. 2012;25(1):5–11.
Ly L, Chan D, Aarabi M, et al. Intergenerational impact of paternal lifetime exposures to both folic acid deficiency and supplementation on reproductive outcomes and imprinted gene methylation. Mol Hum Reprod. 2017;23(7):461–477.
Whidbey C, Harrell MI, Burnside K, et al. A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. J Exp Med. 2013;210(6):1265–1281.
Winram SB, Jonas M, Chi E, Rubens CE. Characterization of group B streptococcal invasion of human chorion and amnion epithelial cells In vitro. Infect immun. 1998;66(10):4932–4941.
Randis TM, Gelber SE, Hooven TA, et al. Group B Streptococcus beta-hemolysin/cytolysin breaches maternal-fetal barriers to cause preterm birth and intrauterine fetal demise in vivo. J Infect Dis. 2014;210(2):265–273.
Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682.
Fitzpatrick M. Measuring cell fluorescence using Image J. In: The Open lab Book. 2014. http://theolb.readthedocs.io/en/latest/imaging/measuring-cell-fluore scence-using-imagej.html
Bock KW. Human and rodent aryl hydrocarbon receptor (AHR): from mediator of dioxin toxicity to physiologic AHR functions and therapeutic options. Biol Chem. 2017;398(4):455–464.
Gennings C, Ellis R, Ritter JK. Linking empirical estimates of body burden of environmental chemicals and wellness using NHANES data. Environ Int. 2012;39(1):56–65.
Weber H, Birnbaum LS. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and 2,3,7,8-tetrachlorodibenzofuran (TCDF) in pregnant C57BL/6 N mice: distribution to the embryo and excretion. Arch Toxicol. 1985;57(3):159–162.
Davies HD, Adair C, McGeer A, et al. Antibodies to capsular polysaccharides of group B Streptococcus in pregnant Canadian women: relationship to colonization status and infection in the neonate. J Infect Dis. 2001;184(3):285–291.
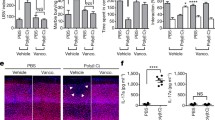
Kothary V, Doster RS, Rogers LM, et al. Group B Streptococcus induces neutrophil recruitment to gestational tissues and elaboration of extracellular traps and nutritional immunity. Front Cell Infect Microbiol. 2017;7:19.
Nguyen DH, Zhou T, Shu J, Mao J. Quantifying chromogen intensity in immunohistochemistry via reciprocal intensity. Cancer InCytes. 2013;2(1):1–4
Ariel I, Goldman-Wohl D, Yagel S, Gazit E, Loewenthal R. Triple paternal contribution to a normal/complete molar chimeric singleton placenta. Hum Reprod. 2017;32(5):993–998.
Malassine A, Frendo JL, Evain-Brion D. A comparison of placental development and endocrine functions between the human and mouse model. Hum Reprod Update. 2003;9(6):531–539.
Surani MA, Barton SC, Norris ML. Influence of parental chromosomes on spatial specificity in androgenetic-parthe-nogenetic chimaeras in the mouse. Nature. 1987;326(6111): 395–397.
Bruner-Tran KL, Resuehr D, Ding T, Lucas JA, Osteen KG. The role of endocrine disrupters in the epigenetics of reproductive disease and dysfunction: potential relevance to humans. Curr Obstet Gynecol Rep. 2012;1(3):116–123.
Akison LK, Nitert MD, Clifton VL, Moritz KM, Simmons DG. Review: alterations in placental glycogen deposition in complicated pregnancies: current preclinical and clinical evidence. Placenta. 2017;54:52-58.
Domingo P, Barquet N, Alvarez M, Coll P, Nava J, Garau J. Group B streptococcal meningitis in adults: report of twelve cases and review. Clin Infect Dis. 1997;25(5): 1180–1187.
Yanai H, Ban T, Taniguchi T. High-mobility group box family of proteins: ligand and sensor for innate immunity. Trends Immunol. 2012;33(12):633–640.
Luu TM, Rehman Mian MO, Nuyt AM. Long-term impact of preterm birth: neurodevelopmental and physical health outcomes. Clin Perinatol. 2017;44(2):305–314.
Nuyt AM, Lavoie JC, Mohamed I, Paquette K, Luu TM. Adult consequences of extremely preterm birth: cardiovascular and metabolic diseases risk factors, mechanisms, and prevention ave-nues. Clin Perinatol. 2017;44(2):315–332.
March of Dimes, PMNCH, Save the children, WHO. Born Too Soon: The Global Action Report on Preterm Birth. Eds CP Howson, MV Kinney, JE Lawn. World Health Organization. Geneva, 2012.
Palomar L, DeFranco EA, Lee KA, Allsworth JE, Muglia LJ. Paternal race is a risk factor for preterm birth. Am J Obstet Gynecol. 2007;197(2):152.e1–152.e7.
Sharma R, Agarwal A, Rohra VK, Assidi M, Abu-Elmagd M, Turki RF. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol. 2015;13:35.
Simhan HN, Krohn MA. Paternal race and preterm birth. Am J Obstet Gynecol. 2008;198(6):644.e1–644.e6.
Stillerman KP, Mattison DR, Giudice LC, Woodruff TJ. Environmental exposures and adverse pregnancy outcomes: a review of the science. Reprod Sci. 2008;15(7):631–650.
Candela S, Ranzi A, Bonvicini L, et al. Air pollution from incin-erators and reproductive outcomes: a multisite study. Epidemiology. 2013;24(6):863–670.
Jurewicz J, Hanke W, Radwan M, Bonde JP. Environmental fac-tors and semen quality. Int J Occup Med Environ Health. 2009; 22(4):305–329.
Mocarelli P, Gerthoux PM, Patterson DG Jr, et al. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect. 2008;116(1):70–77.
Vested A, Ramlau-Hansen CH, Olsen SF, et al. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ Health Perspect. 2013;121(4):453–458.
Lawrence BP, Vorderstrasse BA. New insights into the aryl hydrocarbon receptor as a modulator of host responses to infection. Semin Immunopathol. 2013;35(6):615–626.
Mulero-Navarro S, Fernandez-Salguero PM. New trends in aryl hydrocarbon receptor biology. Front Cell Dev Biol. 2016;4:45.
Abbott BD, Schmid JE, Pitt JA, et al. Adverse reproductive out-comes in the transgenic Ah receptor-deficient mouse. Toxicol Appl Pharmacol. 1999;155(1):62–70.
McCarthy CE, Duffney PF, Wyatt JD, Thatcher TH, Phipps RP, Sime PJ. Comparison of in vitro toxicological effects of biomass smoke from different sources of animal dung. Toxicol In Vitro. 2017;43:76-86.
Spannhake EW, Reddy SP, Jacoby DB, Yu XY, Saatian B, Tian J. Synergism between rhinovirus infection and oxidant pollutant exposure enhances airway epithelial cell cytokine production. Environ Health Perspect. 2002;110:665-670.
Teske S, Bohn AA, Regal JF, Neumiller JJ, Lawrence BP. Activation of the aryl hydrocarbon receptor increases pulmonary neutrophilia and diminishes host resistance to influenza A virus. Am J Physiol lung Cell Mol Physiol. 2005;289(1):L111–L124.
Koumans EH, Rosen J, van Dyke MK, et al. Prevention of mother-to-child transmission of infections during pregnancy: implementation of recommended interventions, United States, 2003–2004. Am J Obstet Gynecol. 2012;206(2):158.e1–15.e11.
Verani JR, Schrag SJ. Group B streptococcal disease in infants: progress in prevention and continued challenges. Clin Perinatol. 2010;37(2):375–392.
Mancuso G, Midiri A, Beninati C, et al. Dual role of TLR2 and myeloid differentiation factor 88 in a mouse model of invasive group B streptococcal disease. J Immunol. 2004;172(10): 6324–6329.
Rosini R, Margarit I. Biofilm formation by Streptococcus agalac-tiae: influence of environmental conditions and implicated virulence factors. Front Cell Infect Microbiol. 2015;5:6.
Becker KW, Skaar EP. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol Rev. 2014; 38(6): 1235–1249.
Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63(6):425–433.
Barboza R, Lima FA, Reis AS, et al. TLR4-mediated placental pathology and pregnancy outcome in experimental malaria. Sci Rep. 2017;7(1):8623.
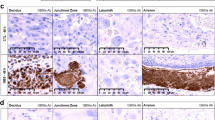
Hayati AR, Mohamed AE, Tan GC. An immunohistochemical study of Toll-like receptors 2 and 4 in placenta with and without infection. Malays J Pathol. 2010;32(1):13–19.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, T., Lambert, L.A., Aronoff, D.M. et al. Sex-Dependent Influence of Developmental Toxicant Exposure on Group B Streptococcus-Mediated Preterm Birth in a Murine Model. Reprod. Sci. 25, 662–673 (2018). https://doi.org/10.1177/1933719117741378
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719117741378