Abstract
Background
Many clinical trials of investigational oncologic agents utilize electrocardiogram (ECG) machine measurements of QTc, for inclusion/exclusion and dosing decisions, though their reliability in this setting has not been established.
Methods
We compared the digital ECG machine QTc measurements with those obtained by a centralized ECG core lab on more than 270,000 consecutive ECGs collected from 299 clinical oncology trials.
Results
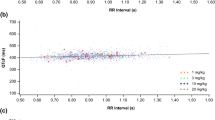
The mean difference between the ECG machine measurements and the central measured QTcF was 1.8 ± 15.7 milliseconds. In addition, 29.7% of ECGs with an ECG machine-measured QTcF >450 milliseconds had a centrally measured QTcF <450 milliseconds, 44.6% of ECGs with an ECG machine-measured QTcF >470 milliseconds had a centrally measured QTcF <470 milliseconds, and 77.2% of ECGs with an ECG machine-measured QTcF >500 milliseconds had a centrally measured QTcF <500 milliseconds. The likelihood of a large discrepancy between the ECG machine- and centrally measured value for QTcF increased at both the high and low ends of the range of ECG machine QTcF measurements.
Conclusions
While on average ECG machine-measured QTcF values were very similar to the central core lab measurements; there were very significant discrepancies which will have important implications for patient recruitment for clinical oncology trials as well as for patient safety during dosing with new oncologic agents. Reliance on ECG machine QTc measurements during clinical oncology trials may lead to unnecessary exclusion of patients as well as unneeded treatment interruptions.
Similar content being viewed by others
References
Shah RR. Can pharmacogenetics help rescue drugs withdrawn from the market? Pharmacogenomics. 2006;7:889–908.
Stockbridge N, Morganroth J, Shah RR, Garnett C. Dealing with global safety issues: was the response to QT-liability of non-cardiac drugs well coordinated? Drug Saf. 2013;36:167–182.
Yap YG, Camm AJ. Drug-induced QT prolongation and torsades de pointes. Heart. 2003;89:1363–1372.
The Clinical Evaluation of QT/QTc Prolongation and Pro-arrhythmic Potential for Non-Antiarrhythmic Drugs: E14. Geneva, Switzerland: International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2005. http://www.ich.org/LOB/media/MEDIA1476.pdf.
Brell J. Prolonged QTc interval in cancer therapeutic drug development: defining arrhythmic risk in malignancy. Prog Cardiovasc Dis. 2010;53:164–172.
Barby J, Pezzullo J, Soignet S. Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol. 2003;21:3609–3615.
Bello CL, Mulay M, Huang X, et al. Electrocardiographic characterization of the QTc interval in patients with advanced solid tumors: pharmacokinetic-pharmacodynamic evaluation of Sunitinib. Clin Cancer Res. 2009;15:7045–7052.
Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551.
Shah R, Morganroth J, Shah DR. Cardiovascular safety of tyrosine kinase inhibitors: with a special focus on cardiac repolarisation (QT interval). Drug Saf. 2013;36:295–316.
Viskin S, Rosovski U, Sands A, et al. Inaccurate electrocardiographic interpretation of long QT: the majority of physicians cannot recognize a long QT when they see one. Heart Rhythm. 2005;2:569–574.
Estes NAM. Computerized interpretation of ECGs: supplement not a substitute. Circ Arrhythm Electrophysiol. 2013;6:2–4.
Mortara Instruments website. www.mortara.com.
Hnatkova K, Gang Y, Batchvarov VN, Malik M. Precision of QT interval measurement by advanced electrocardiographic equipment. Pacing Clin. Electrophysiol. 2006;29:1277–1284.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kleiman, R., Litwin, J. & Morganroth, J. Benefits of Centralized ECG Reading in Clinical Oncology Studies. Ther Innov Regul Sci 50, 123–129 (2016). https://doi.org/10.1177/2168479015597729
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479015597729