Abstract
Vascular endothelial growth factor (VEGF) is an angiogenic mitogen that specifically targets vascular endothelial cells. The objective of this study was to evaluate the role of VEGF in Kawasaki disease (KD), the most common cause of systemic vasculitis in childhood. Serum VEGF levels were measured by ELISA in 22 patients with KD, 22 febrile children with infection, and 19 healthy children. Samples from KD patients were divided into three groups: acute stage (n = 20), subacute stage (n = 13), and convalescent stage (n = 15). The results showed that KD patients in the acute and subacute stages had significantly higher levels of VEGF than did patients with infectious diseases and the healthy control subjects. When compared with the VEGF levels of patients with and without coronary artery lesions (CAL), significantly higher levels of VEGF were observed in the subacute stage in patients with CAL and in patients without CAL in the acute stage. Serial examination revealed that the serum VEGF levels in KD patients with CAL increased from a relatively low level in the acute stage to an extremely high level in the subacute stage. In contrast, patients without CAL were found to have extremely high levels of VEGF only in the acute stage of KD. In KD patients, the serum VEGF levels did not correlate with the inflammatory markers and clinical symptoms. Our results raise the possibility that VEGF is involved in the pathogenesis of KD, especially in the development of CAL. Further study is needed to clarify the biologic effect of VEGF on coronary arteries in KD.
Similar content being viewed by others
Main
VEGF is a multifunctional cytokine that acts as an endothelial cell mitogen and induces microvascular permeability (1–5). VEGF has been shown to play an important role in the pathogenesis of vascular-related diseases, including growth of tumors (5,6), neovascularization in synovial tissue in rheumatoid arthritis (7–9), vascular dysfunction in diabetes mellitus (10), and atherosclerosis of coronary arteries in ischemic heart disease (11,12). KD is the most common cause of systemic vasculitis in childhood, and CAL develop in 10-15% of patients with KD (13). However, the pathogenesis of CAL formation has not yet been elucidated. In this study, we measured the serum levels of VEGF in patients with KD to determine whether VEGF might be involved in the formation of CAL. If a relationship could be shown, then VEGF could be used as a predictor of coronary artery damage, and it could also provide useful information for a new therapy to prevent CAL in KD.
METHODS
Subjects. A total of 63 Japanese children, including 22 KD patients, 22 patients with infectious diseases, and 19 healthy children were enrolled in this study (Table 1). Informed consent was obtained from parent(s) of all subjects who participated in the study. All subjects were seen at the Pediatric Department of Kagoshima University Hospital from April 1995 to May 1997, and serum samples of all KD patients from whom we obtained informed consent during the study period were evaluated.
All KD patients fulfilled the KD criteria as revised by the KD Research Committee of Japan in 1984 (14), and the onset of disease was defined as the day on which the patient developed fever. Echocardiography was performed on all KD patients, and patients with coronary arteries with a diameter of 3 mm (over 5 y of age; 4 mm) or greater were defined as having CAL (15). CAL were found in 5 of 22 patients (23%), and 3 of the 5 patients with CAL eventually developed coronary aneurysms. Forty-eight blood samples from 22 KD patients were divided into three groups: 20 samples from 20 KD patients in the acute stage (within 9 d after onset), 13 samples from 13 patients in the subacute stage (10-20 d after onset), and 15 samples from 15 patients in the convalescent stage (21-50 d after onset). Serial samples were available from 18 patients (8 patients had 3 samples and 10 patients had 2 samples). All patients were treated with a high dose of gammaglobulin and aspirin within 9 d of the onset of disease, and all samples in the acute stage were obtained before the treatment.
Twenty-two age- and sex-matched febrile patients with infections served as disease control subjects (Table 1). Serum levels of CRP in the infectious patients were matched with those of KD patients in the acute stage. Patients with hypoxia were excluded from this study because it has been reported that hypoxia induces production of VEGF in endothelial cells (16).
Nineteen age- and sex-matched afebrile children were studied as healthy control subjects. These children were admitted for selective surgery such as inguinal herniation, or were undergoing investigations for suspected cretinism or other endocrinologic diseases. All the healthy control subjects were afebrile, free of medications, and as investigations showed, normal. All blood samples were centrifuged at 2000 rpm for 15 min, and sera were stored at -80°C until used.
Quantification of VEGF concentration in serum. VEGF levels in all samples were measured in duplicate by a sensitive and highly specific colorimetric ELISA with slight modifications of a chemiluminescence enzyme immunoassay method previously described by Hanatani et al. (17). The recombinant human VEGF121 was purified from the culture medium of the transformed yeast (18). The anti-human VEGF polyclonal antibody was prepared from rabbit serum immunized with the recombinant human VEGF121-glutathione S-transferase fusion protein (19). We purified the IgG fraction from pooled rabbit anti-VEGF serum by ammonium sulfate precipitation and anion exchange chromatography. F(ab′)2 was prepared by digesting the IgG fraction with pepsin and was reduced to Fab′ with 2-mercaptoethylamine. The specificity of this anti-VEGF polyclonal antibody was identified in our previous studies (17,18,20). Ninety-six-well microtiter plates (Combiplate Breakable EB, Labsystems, Helsinki, Finland) were coated with 10 µg/mL purified anti-VEGF antibody in 0.1 M NaCl and 0.25 M carbonate buffer (pH 9.5), 0.1 M NaCl, and 0.1% NaH3. For the assay, 100 µL of samples and serially diluted recombinant human VEGF121 (standards) were added to the wells and incubated for 1 h at 22°C. Then the wells were washed six times, and 100 µL of peroxidase-conjugated Fab′ of the anti-VEGF antibody were added to each well and incubated for 1 h at 22°C. The wells were then washed eight times, and the enzyme reaction was carried out at 22°C for 30 min with o-phenylenediamine (Sigma Chemical Co., St. Louis, MO) as a substrate. The enzyme reaction was terminated by adding 100 µL of 2 M H2SO4 to each well, and the absorbance at 490 nm was measured with a microplate reader (Molecular Devices, Menlo Park, CA). The VEGF content of the samples was estimated from a standard curve determined from the serially diluted VEGF121. Repeated exploratory examinations were used to keep the interassay variability minimal.
Statistical analysis. Results were analyzed using the Mann-Whitney U test for unpaired samples and the Spearman correlation coefficient on the Statview program (Abacus Concepts, Inc., Berkeley, CA). For all tests, p values <0.05 were considered statistically significant.
RESULTS
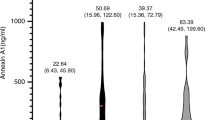
KD patients in the acute and subacute stages showed significant higher levels of serum VEGF than did patients with infectious diseases (p < 0.0005 and p < 0.005, respectively), and healthy control subjects (p < 0.0001 and p < 0.0001, respectively) (Fig. 1). Serum VEGF levels in patients with infectious diseases were significantly higher than those in healthy control subjects (p < 0.005).
The serum levels of VEGF in KD patients and control subjects. *Significant at p < 0.0005 vs infectious diseases and p < 0.0001 vs healthy control subjects. **Significant at p < 0.005 vs infectious diseases and p < 0.0001 vs healthy control subjects. ***Significant at p < 0.005 vs healthy control subjects. ○, KD patients with CAL; •, KD patients without CAL, or control subjects. Horizontal bars indicate median values of VEGF. ID, infectious diseases; HC, healthy control subjects.
KD patients without CAL showed significantly higher levels of serum VEGF than did patients with CAL in the acute stage (p < 0.05) (Table 2). In the subacute stage, however, patients with CAL showed significantly higher levels of serum VEGF than did patients without CAL (p < 0.005). In the convalescent stage, no significant difference was observed in the serum VEGF levels between KD patients with and without CAL.
Furthermore, changes in serum VEGF levels in each patient were studied by using serial serum samples (Fig. 2). The serum VEGF levels in KD patients with CAL increased from a relatively low level in the acute stage (less than 300 pg/mL) to an extremely high level in the subacute stage (more than 400 pg/mL). In contrast, patients without CAL were found to have extremely high levels of VEGF only in the acute stage of KD. Compared with the convalescent stage, significantly higher levels of VEGF were observed in the acute stage in KD patients without CAL (p < 0.005) and in the subacute stage in patients with CAL (p < 0.05).
The serum VEGF levels in infectious patients correlated with serum CRP levels, a clinical marker for inflammation (p < 0.001, r = 0.733) (Fig. 3). In KD patients in the acute stage, however, serum VEGF levels did not correlate with CRP. No other laboratory findings such as white blood cell counts, platelet counts, Hb, IL-6, thrombomodulin, total protein, and electrolytes correlated with serum VEGF levels examined in all KD samples. Also, there was no significant correlation between serum VEGF levels and clinical signs of KD such as duration of fever, presence of injected mucosa, rash, edema, and swollen lymph nodes (data not shown).
DISCUSSION
It has been reported that sera from patients with acute stage KD significantly enhanced proliferation or migration of endothelial cells (21,22). This observation suggests that humoral factors such as cytokines are closely related to the formation of vascular lesions in KD. However, no specific humoral factors are detected in the pathogenesis of CAL in KD patients. VEGF is a newly found cytokine with multiple functions, and it induces endothelial proliferation and microvascular permeability. In the present study, we showed the markedly elevated level of serum VEGF in KD patients. This high concentration of VEGF was sufficient to have some effect on endothelial cells (5).
It has been reported that proinflammatory cytokines such as IL-6 and IL-1β induce the expression of VEGF in various cells (23,24). Therefore, it can be reasoned that patients with inflammatory diseases such as infection or KD have increased levels of VEGF. In our patients with infection, serum VEGF levels were significantly higher than those in healthy control subjects, and the serum VEGF level in infectious patients correlated with the serum CRP level, a clinical marker for inflammation. In the acute stage KD patients, however, serum VEGF levels were much higher than those in patients with infectious diseases, although serum CRP levels of both groups were the same. In addition, KD patients with CAL showed extremely high levels of VEGF in the subacute stage when inflammatory symptoms were alleviated. These findings indicate that the production of VEGF in KD was induced by a factor related to CAL.
VEGF has been thought to have a biphasic effect on endothelial cells, beneficial and harmful. One of the harmful effects of VEGF is inducing damage to endothelial cells. This is compatible with our finding that KD patients with CAL showed extremely high VEGF levels in the subacute stage in which CAL is detected by echocardiography. VEGF has been shown to support transendothelial migration of monocytes, like other proinflammatory cytokines (25). Recent in vitro studies have established that VEGF stimulates endothelial cells to produce NO (26–28). Furthermore, Tilton et al. (10) reported that VEGF mediates vascular dysfunction by producing increased superoxide and NO production. It has also been reported that endogenous NO production is enhanced in KD patients, and that NO plays a role in the development of coronary artery abnormalities in KD (29). Thus, it is possible that VEGF induces CAL in KD patients by promoting inflammation in vessel walls and/or by producing NO at the CAL.
In the subacute stage in KD, we found close relationships between the serum levels of VEGF and nitrite + nitrate, which are final products of NO (data not shown). Investigations of the association between VEGF and NO in KD patients with CAL are in progress.
Alternatively, elevated serum levels of VEGF in KD may be related to the restructuring of damaged vessel walls as a positive feedback mechanism. A remarkably activated system of coagulation and fibrinolysis, caused by endothelial cell damage, has been reported in patients with KD (30). Furthermore, Mohle et al. (31) reported that aggregated platelets may deliver VEGF to injured sites in the vascular system as a first step in vessel repair. In the present study, the KD patients whose serum VEGF levels increased from a low level in the acute stage to a higher level in the subacute stage eventually seemed to develop CAL. This suggests that VEGF has a reparative effect on the damaged coronary artery wall, and patients who failed to produce VEGF in the acute stage are therefore likely to develop CAL.
In conclusion, our data suggested that VEGF was involved in the pathogenesis of KD, especially in the formation of coronary artery aneurysm. VEGF may be a useful clinical predictor for CAL at the initiation of therapy. At present, however, there are no data to prove this possibility directly. Therefore, further in vitro study is currently under way to clarify the role of VEGF in KD.
Abbreviations
- VEGF:
-
vascular endothelial growth factor
- KD:
-
Kawasaki disease
- CAL:
-
coronary artery lesions
- CRP:
-
C-reactive protein
- NO:
-
nitric oxide
REFERENCES
Ferrara N, Henzel WJ 1989 Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 161: 851–858
Leung DW, Cachianes G, Kuang W-J, Goeddel DV, Ferrara N 1989 Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306–1309
Conn G, Soderman D, Schaffer M-T, Wile M, Hatcher V, Thomas K 1990 Purification of a glycoprotein vascular endothelial cell mitogen from a rat glioma cell line. Proc Natl Acad Sci USA 87: 1323–1327
Connolly DT, Heuvelmann DM, Nelson R, Olander JV, Eppley B, Delfino JJ, Siegel RN, Leingruber RS, Feder J 1989 Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest 84: 1470–1478
Ferrara N, Houck K, Jakeman L, Leung DW 1992 Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev 13: 18–32
Plate KH, Breier G, Weich HA, Risau W 1992 Vascular endothelial growth factor is a potential tumor angiogenesis factor in human gliomas in vivo. Nature 359: 845–848
Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong WL, Pope RM, Ferrara N 1994 Vascular endothelial growth factor, a cytokine modulating endothelial function in rheumatoid arthritis. J Immunol 152: 4149–4156
Fava RA, Olsen NJ, Spencer-Green G, Yeo KT, Yeo TK, Berse B, Jackman RW, Senger DR, Dvorak HF, Brown LF 1994 Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med 180: 341–346
Nagashima M, Yoshino S, Ishiwata T, Asano G 1995 Role of Vascular endothelial growth factor in angiogenesis of rheumatoid arthritis. J Rheumatol 22: 1624–1630
Tilton RG, Kawamura T, Chang KC, Ido Y, Bjercke RJ, Stephan CC, Brock TA, Williamson JR 1997 Vascular dysfunction induced by elevated glucose levels in rats is mediated by vascular endothelial growth factor. J Clin Invest 99: 2192–2202
Li J, Brown LF, Hibberd MG, Grossman JD, Morgan JP, Simons M 1996 VEGF, flk-1, and flt-1 expression in a rat myocardial infarction model of angiogenesis. Am J Physiol 270: H1803–H1811
Hashimoto E, Ogita T, Nakaoka T, Matsuoka R, Takao A, Kira Y 1994 Rapid induction of vascular endothelial growth factor expression by transient ischemia in rat heart. Am J Physiol 267: H1948–H1954
Yanagawa H, Yashiro M, Nakamura Y, Kawasaki T, Kato H 1995 Results of 12 nationwide epidemiological incidence surveys of Kawasaki disease in Japan. Arch Pediatr Adolesc Med 149: 779–783
Japan Kawasaki Disease Research Committee 1984 Diagnostic Guidelines of Kawasaki Disease, 4th Rev Ed. Japan Kawasaki Disease Research Committee, Tokyo
Emmanouilides GC, Riemenschneider TA, Allen HD, Gutgesell HP 1995 Moss and Adams' Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult, 5th Ed. Williams & Wilkins, Baltimore, 1390–1399.
Nomura M, Yamagishi S, Harada S, Hayashi Y, Yamashima T, Yamashita J, Yamamoto H 1995 Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes. J Biol Chem 270: 28316–28324
Hanatani M, Tanaka Y, Kondo S, Ohmori I, Suzuki H 1995 Sensitive chemiluminescence enzyme immunoassay for vascular endothelial growth factor/vascular permeability factor in human serum. Biosci Biotech Biochem 59: 1958–1959
Kondo S, Matsumoto T, Yokoyama Y, Ohmori I, Suzuki H 1995 The shortest isoform of human vascular endothelial growth factor (VEGF/VPF121) produced by Saccharomyces cerevisiae promotes both angiogenesis and vascular permeability. Biochim Biophys Acta 1243: 195–202
Kondo S, Asano M, Suzuki H 1993 Significance of vascular endothelial growth factor/vascular permeability factor for solid tumor growth, and its inhibition by the antibody. Biochem Biophys Res Commun 194: 1234–1241
Kondo S, Asano M, Matsuo K, Ohmori I, Suzuki H 1994 Vascular endothelial growth factor/vascular permeability factor is detectable in the sera of tumor-bearing mice and cancer patients. Biochim Biophys Acta 1221: 211–214
Hashimoto Y, Yoshinoya S, Aikawa T, Mitamura T, Miyoshi Y, Muranaka M, Miyamoto T, Yanase Y, Kawasaki T 1986 Enhanced endothelial cell proliferation in acute Kawasaki disease (muco-cutaneous lymph node syndrome). Pediatr Res 20: 943–946
Sakata K, Kita M, Imanishi J, Onouchi Z, Liu Y, Mitsui Y 1995 Effect of Kawasaki disease on migration of human umbilical vein endothelial cells. Pediatr Res 38: 501–505
Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ 1996 Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem 271: 736–741
Li J, Perrella MA, Tsai JC, Yet SF, Hsieh CM, Yoshizumi M, Patterson C, Endege WO, Zhou F, Lee ME 1995 Induction of vascular endothelial growth factor gene expression by interleukin-1β in rat aortic smooth muscle cells. J Biol Chem 270: 308–312
Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, Pan YCE, Olander JV, Connolly DT, Stern D 1990 Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med 172: 1535–1545
Zee R, Murohara T, Lou Z, Zollmann F, Passeri J, Lekutat C, Isner JM 1997 Vascular endothelial growth factor/Vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation 95: 1030–1037
Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC 1997 Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 100: 3131–3139
Tsurumi Y, Murohara T, Krasinski K, Chen D, Witzenbichler B, Kearney M, Couffinhal T, Isner JM 1997 Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nat Med 3: 879–886
Iizuka T, Oishi K, Sasaki M, Hatanaka Y, Minatogawa Y, Uemura S, Koike M 1997 Nitric oxide and aneurysm formation in Kawasaki disease. Acta Paediatr 86: 470–473
Burns JC, Glode MP, Clarke SH, Wiggins JJr, Hathaway WE 1984 Coagulopathy and platelet activation in Kawasaki syndrome: identification of patients at high risk for development of coronary artery aneurysms. J Pediatr 105: 206–211
Mohle R, Green D, Moore MAS, Nachman RL, Rafii S 1997 Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA 94: 663–668
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maeno, N., Takei, S., Masuda, K. et al. Increased Serum Levels of Vascular Endothelial Growth Factor in Kawasaki Disease. Pediatr Res 44, 596–599 (1998). https://doi.org/10.1203/00006450-199810000-00021
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199810000-00021
This article is cited by
-
Markers of Endothelial Dysfunction in Kawasaki Disease: An Update
Clinical Reviews in Allergy & Immunology (2024)
-
Identification of candidate diagnostic serum biomarkers for Kawasaki disease using proteomic analysis
Scientific Reports (2017)
-
The number and function of circulating endothelial progenitor cells in patients with Kawasaki disease
European Journal of Pediatrics (2010)
-
The CCR5 (−2135C/T) Polymorphism may be Associated with the Development of Kawasaki Disease in Korean Children
Journal of Clinical Immunology (2009)
-
Lack of Association of the Vascular Endothelial Growth Factor Gene Polymorphisms with Kawasaki Disease in Taiwanese Children
Journal of Clinical Immunology (2008)