Abstract
Medical optical imaging (MOI) uses light emitted into opaque tissues to determine the interior structure. Previous reports detailed a portable time-of-flight and absorbance system emitting pulses of near infrared light into tissues and measuring the emerging light. Using this system, optical images of phantoms, whole rats, and pathologic neonatal brain specimens have been tomographically reconstructed. We have now modified the existing instrumentation into a clinically relevant headband-based system to be used for optical imaging of structure in the neonatal brain at the bedside. Eight medical optical imaging studies in the neonatal intensive care unit were performed in a blinded clinical comparison of optical images with ultrasound, computed tomography, and magnetic resonance imaging. Optical images were interpreted as correct in six of eight cases, with one error attributed to the age of the clot, and one small clot not seen. In addition, one disagreement with ultrasound, not reported as an error, was found to be the result of a mislabeled ultrasound report rather than because of an inaccurate optical scan. Optical scan correlated well with computed tomography and magnetic resonance imaging findings in one patient. We conclude that light-based imaging using a portable time-of-flight system is feasible and represents an important new noninvasive diagnostic technique, with potential for continuous monitoring of critically ill neonates at risk for intraventricular hemorrhage or stroke. Further studies are now underway to further investigate the functional imaging capabilities of this new diagnostic tool.
Similar content being viewed by others
Main
Current brain-imaging techniques are limited, especially for critically ill neonates. Most existing medical-imaging modalities are not portable, whereas portability may be necessary for those patients who most require urgent or ongoing brain imaging. Such critically ill patients are frequently unstable and unable to tolerate transport to CT or MRI scanner facilities and do not tolerate the repeated scanning necessary to follow an ongoing or evolving condition. In addition, most established imaging techniques require exposure to noxious agents, such as i.v. contrast, radiation, or radioactive emitters. For example, i.v. contrast may cause anaphylaxis or lead to renal failure, a significant risk in a neonate or premature infant. Finally, in the critically ill infant, in whom the cerebral blood flow, blood volume, and brain oxygenation are constantly changing, a continuous imaging technology is crucial if early and specific intervention is to occur, thereby preventing subsequent neuro-developmental disability. Current imaging modalities are not continuous; prolonged ultrasound exposure is neither feasible nor advisable given that the degree of thermal injury to the developing fetus is unknown.
Optical tomography is an evolving technology that includes aspects of both spectroscopy and imaging(1,2). Light in the red and near infrared range (600 nm to 1300 nm) passes through the skull, brain, and other tissues and emerges in small amounts bearing clues about the tissues through which it has passed. Medical optical spectroscopy (MOS) is based upon a characteristic oxygen-dependent absorption of light by Hb and the mithochondrial enzyme cytochrome aa3(3). Other components also have identifiable optical signatures and can be quantitated such as water, glucose, and various tissue pigments. MOS has been investigated for several years to measure cerebral blood volume, cerebral blood flow, and oxygen utilization in the brain(4–9). Medical optical imaging (MOI) is made possible because light passing through tissues is absorbed and scattered to a greater or lesser degree based on chemical and physical composition of the tissue. Historically, optical concentration estimates have been nonquantitative because of the irregular photon paths caused by scattering within tissues; optical images of tissues have therefore been poor. Resolving photon transit time allows separation of the combined effects of the absorbance and scattering. Imaging using time-resolved and frequency-resolved systems has been reported by our group and others(9–21). We have previously reported imaging using near infrared optical tomography of resin model systems and small animals using a tomographic rotating stage-based scanner(17,18). We have described imaging of pathologic specimens of sheep and neonate brains, successfully detecting both intraventricular hemorrhage and subependymal hemorrhage using photon absorbance and scattering data(19). Gopinath et al.(22) have found that optical detection can be used to detect hemorrhage in living adult patients after neurosurgery. We have now converted our scanner to a clinical system based upon an imaging fiber optic headband(20,21) and report here the results of the first in vivo MOI studies in humans with and without structural brain abnormalities. Further, we cross-validate these results by comparison with existing imaging modalities including ultrasound, CT, and MRI.
MATERIALS AND METHODS
Time-of-flight device. We used a portable time-of-flight and absorbance system (TOFA) that emits a pulse of light into an object from multiple positions and detects the photons that are transmitted through the tissue to multiple other positions. The transit time through the tissue for each detected photon was measured, and information from all photons was used to reconstruct an image of the tissue. This system has been previously described(15,17,19) and uses two diode lasers that emit very low intensity light (100 µW average power) at 785-nm and 850-nm wavelengths. The previously reported system used mechanical scanning with a computer-controlled positioning stage and fixed-position emitter fibers, much like a CT scanner, and was impractical for bedside use. Therefore, we modified our scanning system, using a commercially available switching network (DiCon FiberOptic Systems, Berkeley, CA), to allow for a scanning system without other moving parts. A fiber optic headband, which could be placed and secured around an infant's head, was then constructed. The headband consisted of 34 optode pairs separated by a variable distance, in this case approximately 1 cm of fiber to fiber. Each optode pair consisted of a custom-made pair of fibers (Purdy Electronics, InterOptics Division, Burlingame, CA), one for emission and one for sensing, each about 5 m in length. Each fiber terminated in a miniature right angle reflecting prism. The ends of the fibers and prisms were fixed in an optical grade epoxy glue drop and then embedded in a soft neoprene backing to ensure patient comfort and safety (Fig. 1). The headband was constructed in such a way that the flat prism faces directly touched the patient's skin. Photons from each diode laser were routed to the emitter switcher, which, through system software control, were further routed to emitter fibers 1-34 of the fiber optic headband. Transmitted photons were then collected by fibers 1-34, again under the control of system software. To determine a "time zero" for each fiber, the emitter switcher pathway length, the detector switcher pathway length, and the length of the emitter and detector fibers were calibrated for actual photon time delay in picoseconds. This information was stored in an array accessible by the TOFA system software.
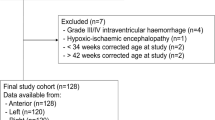
Patient selection. Eight clinical studies were performed under an institutional review board-approved imaging protocol. Premature infants with and without IVH, as well as extracorporeal membrane oxygenation (ECMO) patients, were eligible for the study. Informed consent was obtained before each optical study. Optical scans were collected within a few days of an ultrasound examination, such that the optical findings could be compared with a known standard imaging examination. In all, eight scans were collected from six patients, aged 2 d to 24 d with gestational ages 23 wk to 39 wk. Patients were enrolled over a 4-mo period. Two patients had no IVH by ultrasound, whereas the remaining four patients had unilateral or bilateral IVH by ultrasound. One patient with IVH was optically imaged on three occasions at 4, 6, and 12 d of age.
Scanning. The fiber optic headband, as described above, was placed by a neonatal specialist, carefully avoiding i.v. catheters, ventilator tubing, and diagnostic probes. In full-term infants, the entire headband consisting of 34 fiber pairs would fit around the circumference of the head; however, in small preterm infants, the headband was modified to fit the smaller head circumference by using fewer fiber pairs while maintaining fiber pair spacing at approximately 1 cm of fiber to fiber. Location of each fiber was identified in space by assigning each fiber Cartesian coordinates. These coordinates were converted into polar coordinates and rotated into standard radiology image position by the TOFA software. This software allows an "anterior" fiber label to be placed into the headband positioning file to identify the fiber at the front of the head. In this manner, the fiber headband could be placed in the most convenient and comfortable orientation for the patient, while the image could be rotated or flipped, as needed, to be presented in standard radiographic orientation. Spatial coordinates were confirmed in real time by the user. The infant's head was then scanned using a scan pattern with emission at each fiber location, followed by sequential detection at the remaining fiber locations. Time-of-flight and absorbance curves were collected at each step until a comprehensive circumferential scan of the infant head was completed. On average, scans required several hours to complete, but several infants were monitored for as long as 6 h while serial scans were performed. The initial scans were performed at 785-nm wavelength only, but subsequent scans were performed at both 785 nm and 850 nm.
Image generation and cross-correlation. For each TOFA curve collected, a variety of factors was determined including scattering coefficient, absorption coefficient, average ratio of optical path length to physical emitter-detector separation (known as DPF, or differential path length factor), as well as the rate of change of these variables over space. An image was then reconstructed mathematically using custom-written software, using either scattering or absorption data(18). In this study, the reading of the ultrasound examination was performed by a staff radiologist, who was blinded to the optical results. The reading of the optical scan was performed by one of us (D.A.B.) who was blinded to the ultrasound results and patient histories. The neonatal specialist collecting the optical data (J.V.H.) was not blinded and considered the patient's medical history when evaluating each patient for enrollment to ensure patient appropriateness and stability for examination. Data collection for imaging required 1-6 h. Data image processing required approximately 2-5 min.
RESULTS
Eight optical scans were completed on six infants (one infant was scanned on three different days). A photograph of the headband placed around an infant's head is shown (Fig. 1). The results of the eight optical scans and their correlating ultrasound, CT, and MRI readings are shown (Table 1). Hemorrhages were best seen on images of calculated absorbance, of rate of change in scattering coefficient over distance, and of average distance traveled by light. The optical scans were interpreted as correct in six of eight cases (Table 2). In one case, an optical scan initially read as a false positive bleed on the right side was ultimately read as correct when it was noted that the ultrasound had been mislabeled at an outside hospital with regard to left and right sides. There were no false positive scans; in all scans in which a bleed was seen, a bleed of corresponding grade was seen on ultrasound, CT, or MRI. There were two false negative scans. In the first case, a small bleed was missed on the scan; in the second case, a bleed was not seen on the third of three optical scans of the same infant, although it was clearly visible on earlier scans and remained visible on all three ultrasound examinations.
Four infants are now described, and their optical scans are shown along with the corresponding ultrasound, CT, and MRI scans (Figs. 2-5).
In Figure 2, normal ultrasound and optical examinations are shown. Patient B.B., a 35-wk gestational age infant treated with ECMO for severe septic shock, received a head ultrasound examination on day 2 of life that revealed no hemorrhages. An optical scan on day 2 similarly revealed no bleed. The optical scan and the ultrasound were interpreted as being an agreement.
In Figure 3, small hemorrhages on ultrasound and optical scanning are shown. Patient G.C., a 24-wk gestational age premature infant, received a head ultrasound examination on day 20 of life, which revealed a resolving left germinal matrix hemorrhage. An optical scan on day 20 constructed using scattering data similarly shows an area of increased scattering on the left side in the region of the left-sided hemorrhage identified by head ultrasound. The optical scan and the ultrasound were interpreted as being in agreement.
Comparison of optical and ultrasound scans showing a small hemorrhage. (A) Axial optical tomographic image shows an area of increased scattering on the left side (arrow) in the region of the left-sided hemorrhage identified by head ultrasound. (B) Coronal head ultrasound image reveals a left germinal matrix hemorrhage (arrow). Images of the hemorrhage differ, in part, as they measure along different imaging axes and were obtained at different times.
In Figure 4, large unilateral hemorrhages on ultrasound and optical scanning are shown. Patient B.S., a 24-wk gestational age infant, received a head ultrasound on day 1 of life at an outside hospital, which was interpreted to represent a left grade IVH (outside scan not shown). An optical scan on day 4 of life revealed a large right intraventricular hemorrhage, with no left-sided hemorrhage. However, a repeat head ultrasound examination at our institution on day 6 of life revealed a large right-sided hemorrhage. The optical scan and the ultrasound were interpreted as being in agreement.
Comparison of optical and ultrasound scans showing a large hemorrhage. (A) Axial optical tomographic image shows increased scattering on the right side (arrow), consistent with a clot in that area. (B) Coronal head ultrasound image reveals a large hemorrhage (arrow). Images differ in part as they measure along different imaging axes and were obtained at different times.
In Figure 5, large, bilateral hemorrhages on optical, CT, and MRI scans are shown. Patient B.I., a 39-wk gestational age infant with congenital heart disease, received a head ultrasound on day 8 of life, which revealed a bilateral grade 4 IVH. A head CT on day 8 also revealed bilateral IVH, as did a head MRI on day 15 reveal bilateral induration and blood in the ventricles, consistent with IVH. An optical scan performed at 11 d of age also shows large, bilateral bleeds. The optical scan was interpreted as being in agreement with the CT and MRI.
Comparison of axial optical, MRI, and CT scans showing large hemorrhages. (A) An optical tomographic image, performed at 11 d of age, reveals large bilateral bleeds, consistent with bilateral hemorrhages (arrows). (B) Head CT image of patient B.I. performed at 8 d of age reveals bilateral intraventricular hemorrhages (arrows). (C) Head MRI performed at 15 d of age reveals layering of blood in the lateral ventricles (arrows). Images differ, in part, as they measure different distributions of axial tomographic sections and were obtained at different times.
DISCUSSION
We have shown that cerebral hemorrhage can be successfully detected in the neonate using near infrared optical scanning. Based on these studies, monitoring and imaging of gross brain pathology is clinically feasible using TOFA technology. The demonstrated level of diagnostic accuracy is less than that of ultrasound, but there are advantages to optical scanning that may outweigh the decreased structural resolution, including portability, continuous use, and lack of harmful radiation.
There were several areas of disagreement between the optical scans and the ultrasound examinations in this study. First, the optical, MRI, and ultrasound images of the hemorrhages differ, in part, as they measure different distributions of axial tomographic sections, or were collected at different imaging orientations, and were obtained at different times. Second, in the case of patient B.S., the initial head ultrasound was done at an outside hospital and was marked to indicate a bleed on the left side. However, the optical scan indicated a bleed on the right side. The next head ultrasound, performed at our hospital 5 d later, confirmed the optical scan result that the IVH was indeed on the right side. Therefore, this optical scan is interpreted as giving the correct result.
The third case of disagreement is more complicated, yet more intriguing. Again, in the case of patient B.S., the right-sided IVH appears smaller on the second scan but still is apparent, whereas in the head ultrasound scan the bleed remains plainly visible. By day 12 of life, when a third optical scan was performed, the bleed is no longer evident on the optical scan. We suggest that this may be the result of the blanching to the Hb in the clot as it evolves toward a mature clot. If this hypothesis is correct, this missed bleed does not diminish the ability of the system to detect fresh hemorrhages, which are the type of bleeds that are medically most significant. Further, although this hypothesis regarding the aging of a bleed will need to be studied further by additional in vivo examinations, this result suggests that optical scanning may provide a method of dating a bleed, much as now is done using MRI technology.
Last, in the case of B.P., the optical scan gave a negative result for bleeding, whereas the head ultrasound revealed a small evolving right-sided germinal matrix bleed. We interpreted this scan as a false negative optical scan. One possibility is that, given that this is a prototype system, the system resolution at the time of this patient's optical scan may not have been adequate to reveal this small bleed. A second possibility is that the theoretical limit of resolution may have been reached. Based upon theoretical considerations, image resolution is not expected to exceed 10-20% of the maximum scan depth or not better than 5 to 10 mm at the ventricles of the full-term infant's brain. Resolution of the system is likely to be less than in CT or MRI in any case; in addition, the processing of our optical scans was confined to identifying IVH only for the purposes of this series. Given what is known about the current optical system resolution, it is not expected that small intraparenchymal hemorrhages, for instance, would have been seen using the optical system because of their size. With further resolution of this system, improvements which are already well underway, results of future optical scans are expected to reveal brain structure in even greater detail.
Since Donn et al.(23) first reported that intracranial hemorrhage could be detected by changes in absorption of visible light transilluminated through an infant's head, much research has been done in the area of optically based diagnostic technologies. Red and near infrared photons penetrate deeply, with an absorbance rate of approximately one absorbance event per 5-10 cm of photon travel, resulting in transmission of a large number of photons through tissue and good detectability at long distances. The transmitted photons have been scattered multiple times. The more highly scattered the photons, such as those passing through some tumors, the more time they require to pass through the tissue between emission and detection; the more direct the path of the photon (e.g. the less scattered the light), the sooner the arrival at the detector and the better the resolution upon imaging. As the speed of light in tissue (c divided by the average index of refraction of 1.36) is about 22 cm/ns, and some photons travel many times the average 4 times as far as the physical separation between emission and detection, the resulting photon diffusion wave can be measured over a few nanoseconds after emission of light into tissue, whereas variations in tissue properties produce changes in transit time on the order of picoseconds. Measuring the distribution of such transit times by time-resolved techniques such as TOFA yields information allowing for quantitation of scattering and absorbance using equations that treat photon migration through tissue analogously to other processes involving diffusive transport, such those used to describe the diffusion of heat or gasses. Images may also be formed from such data.
The current resolution of optical infrared imaging is known to be less than that of CT and MRI. Using an algorithm for detectability that we developed and previously reported, the resolution of the system can be estimated to be about 0.3 cm at a depth of 2 cm, and 1.0 cm at a depth of 5 cm(17). However, unlike CT or MRI, our device using time-of-flight technology is portable and, as has been shown here, can easily be used for diagnostic purposes in the critically ill infant at the bedside. All usual procedures may be continued during a TOFA scan, including but not limited to administration of medications, procurement of blood samples, and complete nursing and physician assessments. The ability to positively predict hemorrhage (e.g. the certainty that a hemorrhage is present when seen on optical scanning) may allow for the immediate use of TOFA in postneurosurgical or posttrauma patients, in whom blood accumulation is usually not detected until the patient's condition deteriorates, or use in cardiac bypass patients (such as ECMO), in whom the blood's coagulation time has been altered, resulting in a potentially serious risk for fatal cerebral hemorrhages, which are unpredictable but which may be treatable if detected early.
In CT and MRI, generally considered to be noninvasive diagnostic modalities, i.v. contrast materials are frequently required for these studies. Such agents cannot be used in many critically ill patients, specifically those with renal impairment. Optical imaging currently requires no such contrast agents. However, dyes may also be developed for optical scanning that will extend the range and accuracy of the method, much as such contrast agents have benefited from CT and MRI technologies. For example, indocyanine green has a high molar extinction coefficient, and Chance and co-workers have shown that optical methods can detect and locate less than 10 picomoles of dye buried within a neonatal head-sized tissue phantom. Such optical dyes are likely to be less toxic than radiation-absorbing x-ray or CT contrast agents. indocyanine green, for example, has a long history of use for cardiac output measurement, with minimal renal or hepatic toxicity. Our time-of-flight device could potentially provide continuous bedside monitoring of critically ill, high risk patients; other commonly used diagnostic techniques give only a static "moment in time" view of the brain. This feature, combined with the portability of our device, indicates that optical approaches may be a viable approach to continuously monitor infants undergoing cardiovascular surgery, neurosurgery, and other high-risk procedures that could result in dramatically altered cerebral blood flow.
Although the potential for clinically relevant improvements with MOI has been shown, optical spectroscopy could be combined with optical imaging for an even more powerful diagnostic tool. Measurements of oxyhemoglobin, deoxyhemoglobin, and oxidized cytochrome aa3 at different wavelengths may allow visualization of regional cerebral blood flow and oxygenation with localization of ischemia and hypoxia. Such functional imaging would provide information that would be medically significant regarding the health of tissue, even if the resolution remains relatively low compared with other imaging modalities. Changes in cerebral hemodynamics and oxygenation as measured by nonimaging near infrared spectroscopy have been studied in infants undergoing hypothermic cardiac arrest(24) and have already been shown to be associated with acute hypoxia and neurologic abnormalities(25–27). As these infants are at risk for hypoxic-ischemic brain injury, which is the most important cause of long-term neurodevelopmental disability such as cerebral palsy(28–30), development of this tool for continuous monitoring could be of substantial immediate and future benefit to patients, families, and society. Quantitation of such changes using time-resolved systems may improve the sensitivity or predictiveness of such methods. Using our device, infants at risk for stroke and cerebral hypoxia, including patients undergoing cardiovascular surgery, could be monitored at the bedside. Although information on cerebral perfusion can be obtained with MRI, positron emission tomography, xenon clearance, and Doppler flow studies(28,31,32), such techniques are inadvisable for use in critically ill patients as they are not portable, difficult to perform, involve ionizing radiation, and are static measurements. Changes in cerebral blood flow and oxygenation could be identified at the bedside, early in the course, and as changes are evolving using optical imaging and spectroscopy. In the future, neuroprotective agents could be administered in response to ischemic changes seen on the TOFA bedside monitor. In our group, studies are underway to investigate changes in cerebral blood flow and oxygenation in patients with seizure disorders, cyanotic heart disease, hypoxic-ischemic insult, and those undergoing ECMO therapy and to use optical imaging and spectroscopy as a method to predict brain injury or cerebral palsy.
Abbreviations
- MOI:
-
medical optical imaging
- MOS:
-
medical optical spectroscopy
- TOFA:
-
time-of-flight and absorbance
- CT:
-
computed tomography
- MRI:
-
magnetic resonance imaging
- IVH:
-
intraventricular hemorrhage
- ECMO:
-
extracorporeal membrane oxygenation
References
Benaron DA, Contag C, Stevenson DK, Cheong W-F 1997 Tissue optics. Science 276: 2002–2003.
Benaron DA, Contag C, Contag P 1997 Imaging brain structure and function, infection, and gene expression in the body using light. Phil Trans R Soc Biol Sci B 352: 755–761.
Reynolds EOR, Wyatt JS, Azzopardi D, Delpy DT, Cady EB, Cope M, Wray S 1988 New non-invasive methods for assessing brain oxygenation and haemodynamics. Brit Med Bull 44: 1052–1075.
Brazy JE, Lewis DV, Mitnick MH, Jobsis vander Vliet FF 1985 Non invasive monitoring of cerebral oxygenation in preterm infants: preliminary observations. Pediatrics 75: 217–225.
Edwards AD, Wyatt JS, Richardson C, Potter A, Cope M, Delpy DT, Reynolds EOR 1990 Effects of indomethacin on cerebral haemodynamics in very preterm infants. Lancet 335: 1491–1495.
Jobsis FF 1977 Noninvasive infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198: 1264–1267.
Wyatt JS, Cope M, Delpy DT, Wray S, Reynolds EOR 1986 Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet 2: 1063–1065.
van Houten JP, Cheong W-F, Kermit EL, Machold TR, Stevenson DK, Benaron DA 1996 Clinical measurement of brain oxygenation and function using light-based optical tomography. Pediatr Res 39: 382A
Hintz SR, Benaron DA, Cheong W-F, Stevenson DK 1997 Bedside imaging of motor cortex activation using optical tomography. 41: 291A
Hebden JC, Kruger RA 1990 Transillumination imaging performance: a time-of-flight imaging system. Med Phys 17: 351–356.
Sevick EM, Burch CL, Chance B 1994 Near-infrared optical imaging of tissue phantoms with measurement in the change of optical path lengths. Adv Exp Med Biol 345: 815–823.
Wang L, Ho PP, Liu C, Zhang G, Alfano RR 1991 Ballistic 2-D imaging through scattering walls using an ultrafast optical Kerr gate. Science 253: 769–771.
Andersson-Engels S, Berg R, Svanberg S 1990 Time-resolved transillumination for medical diagnostics. Optical Lett 15: 1179–1181.
Wist AO, Moon P, Meiksin Z, Herr SL, Fatouros PP 1993 High resolution light imaging system for teeth and tissue. J Clin Laser Med Surg 11: 313–321.
Benaron DA, Stevenson DK 1993 Optical time-of-flight and absorbance imaging of biologic media. Science 259: 1463–1466.
Benaron DA, Lenox MA, Stevenson DK 1992 2-D and 3-D images of thick tissue using time-constrained times-of-flight and absorbance spectrophotometry. SPIE 1641: 35–45.
Benaron DA, Ho DC, Spilman S, van Houten JP, Stevenson DK 1994 Tomographic time-of-flight optical imaging device. Adv Exp Med Biol 361: 207–214.
Benaron DA, Ho DC, Spilman S, van Houten JP, Stevenson DK 1994 Non-recursive linear algorithms for optical imaging in diffusive media. Adv Exp Med Biol 361: 215–222.
van Houten JP, Benaron DA, Spilman S, Stevenson DK 1996 Imaging brain injury using time-resolved near infrared light scanning. Pediatr Res 39: 470–476.
Benaron DA, van Houten JP, Cheong W-F, Kermit EL, King RA 1995 Early clinical results of time-of-flight optical tomography in a neonatal intensive care unit. SPIE 2389: 582–596.
Benaron DA, van Houten JP, Cheong W-F, Kermit EL, King RA 1996 Early clinical results of time-of-flight optical tomography in a neonatal intensive care unit. In: Verga Scheggi AM, Martellucci S, Chester AN, Pratesi R (eds) Biomedical Optical Instrumentation and Laser-Assisted Biotechnology NATO, ASI, 305–324.
Gopinath SP, Robertson CS, Grossman RG, Chance B 1993 Near-infrared spectroscopic localization of intracranial hematomas. J Neurosurg 79: 43–47.
Donn SM, Sharp MJ, Kuhns LR, Uy JO, Knake JE, Duchinsky BJ 1979 Rapid detection of neonatal intracranial hemorrhage by transillumination. Pediatrics 64: 843–847.
Kurth CD, Steven JM, Nicholson SC, Chance B, Delivoria-Papdopoulos M 1992 Kinetics of cerebral deoxygenation during deep hypothermic circulatory arrest in neonates. Anesthesiology 77: 656–661.
Wyatt JS, Cope M, Delpy DT, Richardson CE, Edwards AD, Wray S, Reynolds EOR 1990 Quantitation of cerebral blood volume of human infants by near-infrared spectroscopy. J Appl Physiol 68: 1086–1091.
Brazy JE, Lewis DV 1986 Changes in cerebral blood volume and cytochrome aa3 during hypertensive peaks in preterm infants. J Pediatr 108: 983–987.
van Bel P, Dorrepaal CA, Benders M, Zeeuwe P, van de Bor M, Berger HM 1993 Changes in cerebral hemodynamics and oxygenation in the first 24 hours after birth asphyxia. Pediatrics 92: 365–372.
Wyatt JS, Edwards AD, Azzopardi K, Reynolds EOR 1989 Magnetic resonance and near infrared spectroscopy for investigation of perinatal hypoxic-ischaemic brain injury. Arch Dis Child 64: 953–963.
Levene ML, Kornberg J, Williams THC 1985 The incidence and severity of post-asphyxial encephalopathy in full-term infants. Early Hum Dev 11: 21–26.
Mulligan JC, Painter MJ, O'Donoghue PA, MacDonald HM, Allen AC, Taylor PM 1980 Neonatal asphyxia: II. neonatal mortality and long-term sequelae. J Pediatr 96: 903–907.
Skov L, Pryds O, Greisen G 1991 Estimating cerebral blood flow in newborn infants: comparison of near infrared spectroscopy and 133Xe clearance. Pediatr Res 30: 570–573.
Volpe JJ 1995 Specialized studies in the neurological evaluation. In: Volpe JJ (ed) Neurology of the Newborn. WB Saunders, Philadelphia, 149–162.
Author information
Authors and Affiliations
Additional information
This work was supported by the Walter and Idun Berry Fellowship at Stanford, National Institutes of Health Grants N43-NS-4-2315, N43-NS-6-2315, and MOL-RR00070, Office of Naval Research Grant N-00014-94-1024, and the United Cerebral Palsy Foundation. D.A.B. is the Ethel Hausman Fellow of the United Cerebral Palsy Foundation.
Rights and permissions
About this article
Cite this article
Hintz, S., Cheong, WF., Van Houten, J. et al. Bedside Imaging of Intracranial Hemorrhage in the Neonate Using Light: Comparison with Ultrasound, Computed Tomography, and Magnetic Resonance Imaging. Pediatr Res 45, 54–59 (1999). https://doi.org/10.1203/00006450-199901000-00009
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199901000-00009
This article is cited by
-
Neonatal NIRS monitoring: recommendations for data capture and review of analytics
Journal of Perinatology (2021)
-
Diffuse optical tomography to investigate the newborn brain
Pediatric Research (2017)
-
Optical tomography of the neonatal brain
European Radiology (2007)