Abstract
High Pco2 levels attenuate reperfusion injury and ventilation-induced injury in isolated and perfused lungs. We asked whether premature lambs could tolerate 6 h of ventilation with a Pco2 >80 mm Hg and whether the high Pco2 modulated the ventilator-induced injury. Preterm surfactant-treated lambs were ventilated for 30 min with a high tidal volume (Vt) to induce lung injury. The lambs then were ventilated for 5.5 h with a Vt of 6–9 mL/kg to achieve a Pco2 of 40–50 mm Hg in the control group. CO2 was added to the ventilator circuit of a high Pco2 group to maintain an average Pco2 of 95 ± 5 mm Hg. The high Pco2 lambs had heart rates, blood pressures, plasma cortisol values, and oxygenation equivalent to the control lambs. The lungs of the high Pco2 group had significantly higher gas volumes and had less lung injury by histopathology. Indicators of inflammation (white blood cells, hydrogen peroxide production, and IL-1β and IL-8 cytokine mRNA expression in cells from the alveolar wash) qualitatively indicated less injury in the high Pco2 group, although the differences were not significant. Preterm lambs tolerated a very high Pco2 without physiologic compromise for 6 h. The high Pco2 may attenuate ventilator-induced lung injury in the preterm.
Similar content being viewed by others
Main
BPD in preterm infants is highly associated with the use of mechanical ventilation and supplemental oxygen (1, 2). Efforts to decrease the incidence of BPD have included attempts to avoid mechanical ventilation with the use of continuous positive airway pressure (3), different techniques for mechanical ventilation such as high-frequency oscillatory ventilation (4), and lower Vt ventilation resulting in higher Pco2 values (5). In the mature lung, ventilator-mediated injury increases if the lung is inadequately inflated at end-expiration or overinflated at end-inspiration (6, 7). Attempts to decrease lung distention by decreasing Vt will result in increases in Pco2, referred to as “permissive hypercapnia”(8). Laffey et al.(9) recently extended this concept to “therapeutic hypercapnia” by demonstrating that reperfusion injury of the lung can be prevented if the injury and reperfusion occur in the presence of a Pco2 of 80 mm Hg. This work demonstrates that high Pco2 can protect the lung from reperfusion injury, most likely via attenuation of free radical damage. This effect has also been reported for reperfusion injury of other organ systems, such as the brain and heart in adult and newborn animal models (10, 11). Broccard et al.(12) recently reported that high Pco2 also minimized ventilator-induced lung injury in isolated and perfused lungs. However, a protective effect of high Pco2 values has not been demonstrated for ventilator-induced injury in vivo in animals or for the preterm lung. Therefore, we ventilated surfactant-treated preterm lambs to achieve normal Pco2 values or equivalently ventilated other lambs with supplemental CO2 to evaluate the tolerance of the preterm to high Pco2 and the effect of the high Pco2 on indicators of lung injury.
METHODS
Delivery and ventilation of lambs.
The protocol was approved by the Animal Care Use Committee of the Cincinnati Children's Hospital Research Foundation. Anesthetized pregnant Suffolk ewes at 130–132 d gestational age (term = 150 d) were delivered by cesarean section as previously described (13). Each lamb was intubated, fetal lung fluid was aspirated, and 100 mg/kg of a surfactant containing human recombinant surfactant protein C and phospholipids (Venticute, Byk Gulden, Konstanz, Germany) was administered before the first breath (14). This surfactant has been shown to be as effective as natural surfactants and does not independently induce inflammation (15). Each lamb was randomized to a high Pco2 group or a control group. The lambs were ventilated in a pressure-control mode with pressure-limited, time-cycled ventilators (16). The high Pco2 group had supplemental CO2 bled into the ventilator circuit to maintain an arterial Pco2 of >80 mm Hg. The control group was ventilated to a Pco2 of 40–50 mm Hg. The initial ventilator settings were as follows: 40 breaths per minutes, target Vt of 12–15 mL/kg with a pressure limit of 40 cm H2O, a PEEP of 4 cm H2O, an inspiratory time of 0.6 s, and a fraction of inspired oxygen (Fio2) of 100%. Vt were monitored continuously (16, 17). The high Vt was maintained for 30 min to produce a mild lung injury. The Vt was then lowered to 6–9 mL/kg for 5.5 h with a maximum allowable PIP of 35 cm H2O. The Fio2 was adjusted to maintain a Po2 of 100–150 mm Hg. Cord blood and arterial blood were analyzed for white blood cell numbers and differential counts and plasma cortisol levels (16). All animals were sedated with intramuscular injections of ketamine and acepromazine to suppress any spontaneous respirations and no neuromuscular blockade was used. At 6 h, the lambs were deeply anesthetized with pentobarbital (25 mg/kg i.v.), ventilated briefly with 100% oxygen, and the airway was occluded to permit oxygen absorption.
Analyses.
The deflation limb of the pressure-volume curve was measured (17). The left lung was used for an alveolar wash (18). The washes were pooled and saved for saturated phosphatidylcholine (Sat PC) analysis (19), total protein (20), cell counts and differentials, and hydrogen peroxide (H2O2) assay (21). The right upper lobe was inflation fixed in 10% formalin at 30 cm H2O pressure (22). Total RNA was isolated from lung tissue and cell pellets from alveolar wash (22). IL1-β, IL-4, IL-6, IL-8, and tumor necrosis factor-α mRNA were quantified using ribonuclease protection assays with the ribosomal mRNA L32 as a reference RNA (22). Flow cytometry was performed to assess the percentage of cells that were apoptotic using propidium iodide/FITC staining (BD PharMingen, San Diego, CA, U.S.A.) (23). Cells were also incubated with MAb (Serotec, Oxford, UK) against CD11b (αM subunit of integrin CR3), CD14 (receptor for complex of lipopolysaccharide binding protein), and CD44 (proteoglycan link protein) and assayed by flow cytometry (23). The right upper lobe was airway inflation fixed with formalin at a pressure of 30 cm H2O. The amount of inflammation in the right upper lobe was graded in a blinded fashion on three 5-μm sections for each animal (24).
Data analysis.
Student's two-tailed unpaired t tests were used to compare the two groups. Significance was accepted at p < 0.05.
RESULTS
Description of lambs.
One lamb randomized to the high Pco2 group was excluded because it had a congenital diaphragmatic hernia and could not be ventilated. The remaining eight control and six high Pco2 animals had similar birth weights, cord pH, and Pco2 levels at delivery (Table 1). Blood pressures and heart rates for the animals with the high Pco2 levels were similar to controls at 3 and 6 h. There were no differences in the peripheral white blood cell counts at 0, 3, and 6 h between high Pco2 and control groups. Cortisol values also were similar at 0, 3, and 6 h.
Respiratory outcomes.
Although the target Vt during the first 30 min of ventilation was 12–15 mL/kg, the average Vt was 10.8 ± 0.7 mL/kg for the control group and 12.1 ± 0.3 mL/kg for the high Pco2 group because most of the animals were at the maximum allowable PIP of 40 cm H2O (Figs. 1, A and B). This trend toward a Vt difference would bias the results toward the null hypothesis. The Vt was decreased to the target range of 6–9 mL/kg after 30 min. There were no significant differences between the groups for Vt or PIP over the first 30 min or for the subsequent 5.5 h of ventilation.
Sequential measurements of tidal volume (Vt) and peak inspiratory pressure (PIP) during ventilation in premature lambs. The hatched area indicates the first 30 min of high Vt to induce lung injury. (A) Vt was similar in both groups throughout the period of ventilation. (B) The PIP used to achieve the similar Vt for both groups was not different. Values are means ± SE.
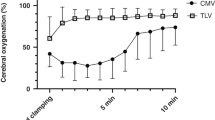
The Pco2 values of the high Pco2 group were maintained at >80 mm Hg using between 0.125 and 1.5 L/min supplemental CO2 in the ventilator circuit (Fig. 2A). The average Pco2 over the 6 h of ventilation was 95 ± 5 mm Hg for the high Pco2 group and was maintained between 40 and 50 mm Hg for the control group by study design. The pH reflected the Pco2 difference throughout the experiment (Fig. 2B). The base deficit for the control group averaged −2.5 ± 1.3 and for the high Pco2 group averaged −6.4 ± 1.3, values that were not different. Oxygenation, as reflected by the a/A ratio, was not different between groups throughout the experiment (Fig. 2C). The compliance for the control group averaged 0.35 ± 0.03 mL/cm H2O/kg and, for the high Pco2 group, averaged 0.42 ± 0.04 mL/cmH2O/kg (p = 0.17) (Fig. 3A). The volumes measured by the deflation pressure volume curves of the lungs were higher for the high Pco2 group than for the control animals (Fig. 3B).
Sequential measurements of Pco2, pH, and a/A ratio during the ventilation of the preterm lambs. (A) Pco2 was higher in the high Pco2 group by study design. (B) The pH was reflective of the Pco2 difference throughout the ventilation period. (C) Oxygenation, as evaluated by the a/A ratio, was not different between the two groups throughout the ventilation period. The a/A ratio was calculated as Pao2/(Pio2 − Paco2) = Pao2/[Fio2 (PB − PH2O) − Paco2]. *p < 0.05 versus the control group. Values are means ± SE.
Compliance and pressure-volume curves. (A) Sequential measurements of compliance throughout the ventilation period. There were no differences in compliance between the groups. (B) Deflation limbs of the pressure volume curves. After 6 h of ventilation there was a significant difference between the high Pco2 group and the control group. *p < 0.05 versus the control group. Values are means ± SE.
Indicators of lung inflammation.
The alveolar wash from the high Pco2 group had a lower total protein, but the difference was not statistically significant (Table 2). Both the total white blood cell counts and the neutrophil counts in the alveolar washes tended to be lower in the high Pco2 group. The hydrogen peroxide production by the cells in the alveolar wash for the high Pco2 group was half of the control. There were no differences in mean fluorescence units for CD11b, CD14, or CD44 expression on cells in the alveolar wash between groups. There was also no difference between the percentage of apoptotic cells per kilogram for the two groups.
Lung injury scores from the histopathology are shown in Table 2. There was no difference for the lung tissue between the control and high Pco2 groups. There was a significant decrease in the score for inflammatory cells in the air spaces for the high Pco2 group compared with the control. Neither group demonstrated severe edema, hemorrhage, or atelectasis.
The levels of cytokine mRNA for IL-1β, IL-4, IL-6, IL-8, and tumor necrosis factor-α from lung tissue were evaluated for the two groups and were not different (Table 3). The cytokine mRNA levels for IL-1β and IL-8 from the cells in the alveolar wash were lower for the high Pco2 group than the control group although the differences were not significant (Table 3). IL-6 mRNA was not detected in cells from the alveolar wash for either group of animals.
DISCUSSION
This study was designed to test the effects of a high Pco2 in a premature animal model of ventilator-induced lung injury. Our endpoints were the assessment of the physiologic tolerance of a high Pco2 and alterations in lung function or inflammation. We found no physiologic instability as reflected by blood pressure or heart rate with the high Pco2. We did find an overall trend toward decreased inflammation and lung injury. The mean deflation pressure-volume curve for the high Pco2 animals retained more gas volume and the inflammation score for the airspace was less in the high Pco2 group. The other indicators of inflammation and injury (total protein in the alveolar washes, white blood cells in alveolar washes, hydrogen peroxide production by alveolar cells, and proinflammatory cytokine expression by alveolar cells) were qualitatively lower in the high Pco2 group.
We chose the preterm lamb for these studies because we could evaluate both physiology and multiple indicators of inflammation and injury in this large animal model. We have characterized the proinflammatory response to mechanical ventilation of preterm lambs (16). For this study, we avoided a severe lung injury by treating with surfactant, but we initially used a high Vt and high pressures for 30 min to cause a mild injury in both groups of animals. The subsequent ventilation was designed to cause minimal further injury by using low Vt and avoiding hyperventilation of the low Pco2 group (17). By matching the ventilation using Vt to adjust pressures, both groups of animals were exposed to the same amount of mechanical ventilation under virtually identical conditions except for the supplemental CO2 received by the high Pco2 group. Therefore, we were able to successfully isolate the Pco2 as the single variable between the two groups of animals. We did not attempt to correct the respiratory acidosis, because pH correction prevented the beneficial effects of high Pco2 in the lung reperfusion injury model (25).
Permissive hypercapnia is being evaluated as a way to avoid the barotrauma required to normalize Pco2 values in ventilated adults with severe lung injury (26, 27). Low Pco2 values have been associated with an increased risk of BPD in ventilated preterm infants (28), but high Pco2 values increase cerebral blood flow and could be a risk factor for intraventricular hemorrhage in the preterm infant (29). Nevertheless, several small trials have randomized ventilated preterm infants to lower versus higher Pco2 targets and report trends consistent with some protection from BPD in the higher Pco2 groups (5, 30). Although many preterm infants with BPD will have chronic elevations of Pco2 into the 50–60 mm Hg range and a few will maintain higher Pco2 levels, there is no clinical information about the safety of high Pco2 values soon after delivery and early in the course of respiratory failure in the preterm. This experiment was designed to be an extreme test of the ability of the preterm to cope with very high Pco2 levels.
The adult human or animal tolerates the respiratory acidosis associated with an acute increase in Pco2 by increasing catecholamines and maintaining cardiac output (31). These preterm lambs, with Pco2 values that averaged 95 mm Hg, had no changes in heart rate or blood pressure and cortisol levels did not change over 6 h. We did not measure catecholamines, although they would be expected to be very high after preterm delivery (32). A limitation of this study is that we did not evaluate cardiac output or other organ system function. In a previous study evaluating low Vt for the initiation of mechanical ventilation in preterm lambs, lambs with Pco2 values of about 90 mm Hg in the first 30 min of life had a 1.5-fold increase in cardiac output and a 5-fold increase in cerebral blood flow relative to lambs with normal Pco2 values (13).
Another concern about high Pco2 levels in the immediate newborn period is that the high pulmonary artery pressure characteristic of the fetal lung would be maintained after birth in the high Pco2-low pH environment (33). Low pH and high Pco2 values have been reported to interfere with the normal vasodilatation of the pulmonary vasculature that occurs after birth (34). Although we did not measure pulmonary artery pressures, oxygenation was not different in these surfactant-treated lambs. Overall postnatal adaptation was essentially equivalent despite the very high Pco2 values.
Our second question was to ask whether “therapeutic hypercapnia” would decrease lung injury. High Pco2 levels decrease multiple indicators of lung injury after ischemia-reperfusion injury (9), and correction of the respiratory acidosis with bicarbonate abrogates the protective effect of the high Pco2(35). High Pco2 in the range of 55 mm Hg also protects the newborn rat brain from injury after unilateral carotid ligation and hypoxia (36). The high Pco2 protects the brain by increasing perfusion and maintaining high energy metabolite levels. High Pco2 also can protect the isolated heart from reperfusion injury (11). The common theme is that the oxidant-mediated ischemia-reperfusion injury cascade is blunted by high Pco2 values. High Pco2 values also can protect the isolated and perfused rabbit lung from ventilator-induced lung injury (12). However, CO2 effects on ventilator-mediated lung injury in the whole animal have not been reported. We evaluated a number of the variables that have been associated with lung injury in ventilated adult and preterm animals (16, 37). We were able to show a statistically significant difference between the pressure-volume curves of the two groups and the inflammatory cell infiltration in the airspaces. It is interesting that we could show a difference in the infiltration of inflammatory cells into the airspaces, but not into the lung tissue. One possibility is a small difference in cell number is more apparent in the airspaces than in lung tissue sections. Another consideration is the physiologic action of the high CO2 level: does it exert a systemic effect decreasing inflammatory signaling and cell migration, or does it act locally, disabling the inflammatory cells already found in the lungs? With the group size that we used, we did not demonstrate definitively that the high Pco2 levels protected the lungs from the inflammatory mediators that appear with the initiation of mechanical ventilation of the preterm lung. However, the directional changes of all the variables that we measured favored the high Pco2 group.
Recent clinical reports of the benefits of a more conservative approach to the management of the preterm lung emphasize avoiding intubation in the delivery room and the use of continuous positive airway pressure to avoid mechanical ventilation (38). A result of such an approach is that the infant will have higher Pco2 values (39). Our results demonstrate no measured adverse effects of extremely high Pco2 levels. However, this study does not address any potential benefits of the levels of elevated Pco2 that occur clinically, nor does it address a number of potential injuries that might be caused by elevated Pco2 values in the preterm.
Abbreviations
- BPD:
-
bronchopulmonary dysplasia
- Vt:
-
tidal volume
- PIP:
-
peak inspiratory pressure
- PEEP:
-
positive end-expiratory pressure
References
O'Brodovich HM, Mellins RB 1985 Bronchopulmonary dysplasia. Unresolved neonatal acute lung injury. Am Rev Respir Dis 132: 694–709
Jobe A, Bancalari E 2001 NICHD/NHLBI/ORD Workshop summary: bronchopulmonary dysplasia. Am J Respir Crit Care Med 163: 1723–1729
Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, Bertelsen A, Agertoft L, Djernes B, Nathan E, Reinholdt J 1999 Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks' gestation. Pediatrics 103: E24
Gerstmann DR, Minton SD, Stoddard RA, Meredith KS, Monaco F, Bertrand JM, Battisti O, Langhendries JP, Francois A, Clark RH 1996 The Provo multicenter early high-frequency oscillatory ventilation trial: improved pulmonary and clinical outcome in respiratory distress syndrome. Pediatrics 98: 1044–1057
Mariani G, Cifuentes J, Carlo WA 1999 Randomized trial of permissive hypercapnia in preterm infants. Pediatrics 104: 1082–1088
Muscedere JG, Mullen JBM, Gan K, Slutsky AS 1994 Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149: 1327–1334
Dreyfuss D, Saumon G 1998 Ventilator-induced lung injury. Am J Respir Crit Care Med 157: 294–323
Feihl F, Perret C 1994 Permissive hypercapnia. Am J Respir Crit Care Med 150: 1722–1737
Laffey JG, Tanaka M, Engelberts D, Luo X, Yuan S, Tanswell AK, Post M, Lindsay T, Kavanagh BP 2000 Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med 162: 2287–2294
Vannucci RC, Brucklacher RM, Vannucci SJ 1997 Effect of carbon dioxide on cerebral metabolism during hypoxia-ischemia in the immature rat. Pediatr Res 42: 24–29
Nomura F, Aoki M, Forbess JM, Mayer JE 1994 Effects of hypercarbic acidotic reperfusion on recovery of myocardial function after cardioplegic ischemia in neonatal lambs. Circulation 90: 321–327
Broccard AF, Hotchkiss JR, Vannay C, Markert M, Sauty A, Feihl F, Schaller MD 2001 Protective effects of hypercapnic acidosis on ventilator-induced lung injury. Am J Respir Crit Care Med 164: 802–806
Wada K, Jobe AH, Ikegami M 1997 Tidal volume effects on surfactant treatment responses with the initiation of ventilation in preterm lambs. J Appl Physiol 83: 1054–1061
Davis AJ, Jobe AH, Häfner D, Ikegami M 1998 Lung function in premature lambs and rabbits treated with a recombinant SP-C surfactant. Am J Respir Crit Care Med 157: 553–559
Ikegami M, Jobe A 2002 Injury responses to different surfactants in ventilated premature lamb lungs. Pediatr Res 51: 689–695
Naik AS, Kallapur SG, Bachurski CJ, Jobe AH, Michna J, Kramer BW, Ikegami M 2001 Effects of ventilation with different positive end-expiratory pressures on cytokine expression in the preterm lamb lung. Am J Respir Crit Care Med 164: 494–498
Michna J, Jobe AH, Ikegami M 1999 Positive end-expiratory pressure preserves surfactant function in preterm lambs. Am J Respir Crit Care Med 160: 634–639
Jobe A, Ikegami M, Jacobs H, Jones S, Conaway D 1983 Permeability of premature lamb lungs to protein and the effect of surfactant on that permeability. J Appl Physiol 55: 169–176
Mason RJ, Nellenbogen J, Clements JA 1976 Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res 17: 281–284
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ 1951 Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
Kramer BW, Moss TJ, Willet KE, Newnham JP, Sly PD, Kallapur SG, Ikegami M, Jobe AH 2001 Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med 164: 982–988
Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski C 2001 Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol 280: L527–L536
Kramer BW, Jobe AH, Bachurski CJ, Ikegami M 2001 Surfactant protein A recruits neutrophils into the lungs of ventilated preterm lambs. Am J Respir Crit Care Med 163: 158–165
Jobe AH, Newnham JP, Willet KE, Sly P, Ervin MG, Bachurski C, Possmayer F, Hallman M, Ikegami M 2000 Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol 182: 401–408
Laffey JG, Engelberts D, Kavanagh BP 2000 Injurious effects of hypocapnic alkalosis in the isolated lung. Am J Respir Crit Care Med 162: 399–405
Amato MBP, Barbas CSV, Medeiros DM, Magaldi RB, Schettino GPP, Lorenzi-Filho G, Kairalla RA, et al 1998 Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338: 347–354
Brochard L, Roudot-Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez-Mondejar E, Clementi E, Mancebo J, Factor P, Matamis D, Ranieri M, Blanch L, Rodi G, Mentec H, Dreyfuss D, Ferrer M, Brun-Buisson C, Tobin M, Lemaire F 1998 Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume Reduction in ARDS. Am J Respir Crit Care Med 158: 1831–1838
Garland JS, Buck RK, Allred EN, Leviton A 1995 Hypocarbia before surfactant therapy appears to increase bronchopulmonary dysplasia risk in infants with respiratory distress syndrome. Arch Pediatr Adolesc Med 149: 617–622
Szymonowicz W, Yu VY, Wilson FE 1984 Antecedents of periventricular haemorrhage in infants weighing 1250 g or less at birth. Arch Dis Child 59: 13–17
Carlo WA, Stark AR, Wright LL, Tyson JE, Papile LA, Shankaran S, Donovan EF, Oh W, Bauer CR, Saha S, Poole WK, Stoll B 2002 Minimal ventilation to prevent bronchopulmonary dysplasia in extremely-low-birth-weight infants. J Pediatr 141: 370–374
Tuxen DV 1994 Permissive hypercapnic ventilation. Am J Respir Crit Care Med 150: 870–874
Padbury JF, Polk DH, Newnham JP, Lam RW 1985 Neonatal adaptation: greater sympathoadrenal response in preterm than full-term fetal sheep at birth. Am J Physiol 248: E443–E449
Rudolph AM 1980 High pulmonary vascular resistance after birth: I. Pathophysiologic considerations and etiologic classification. Clin Pediatr 19: 585–590
Lyrene RK, Philips JB 1984 Control of pulmonary vascular resistance in the fetus and newborn. Clin Perinatol 11: 551–564
Laffey JG, Engelberts D, Kavanagh B P 2000 Buffering hypercapnic acidosis worsens acute lung injury. Am J Respir Crit Care Med 161: 141–146
Vannucci RC, Towfighi J, Heitjan DF, Brucklacher RM 1995 Carbon dioxide protects the perinatal brain from hypoxic-ischemic damage: an experimental study in the immature rat. Pediatrics 95: 868–874
Tremblay LN, Slutsky AS, Dreyfuss D, Saumon G 1998 Ventilator-induced lung injury: mechanisms and clinical correlates. In: Marini, JJ Slutsky AS (eds) Physiological Basis of Ventilatory Support. Marcel Dekker, New York, 395–451.
Van Marter LJ, Allred EN, Pagano M, Sanocka U, Parad R, Moore M, Susser M, Paneth N, Leviton A 2000 Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network. Pediatrics 105: 1194–1201
Lindner W, Vossbeck S, Hummler H, Pohlandt F 1999 Delivery room management of extremely low birth weight infants: spontaneous breathing or intubation?. Pediatrics 103: 961–967
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grant DH-12714 from the National Institute of Child Health and Human Development.
Rights and permissions
About this article
Cite this article
Strand, M., Ikegami, M. & Jobe, A. Effects of High Pco2 on Ventilated Preterm Lamb Lungs. Pediatr Res 53, 468–472 (2003). https://doi.org/10.1203/01.PDR.0000049463.76133.8F
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000049463.76133.8F
This article is cited by
-
Hypercapnic acidosis in ventilator-induced lung injury
Intensive Care Medicine (2010)
-
Does Hypercapnia Ameliorate Hyperoxia-Induced Lung Injury in Neonatal Rats?
Lung (2010)