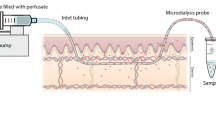
Abstract
It is common to refer to microdialysis as a minimally invasive procedure, likening it to insertion of an artificial capillary. While a comparison of this type allows the process to be easily visualized by those outside the field, it tends to provide a false impression of the localized perturbation of the tissue space that is caused by catheter insertion. With the increased acceptance of microdialysis sampling as a viable in vivo sampling method, many researchers have begun to use the technique to explore inflammatory and immune-mediated diseases in the skin and other organs. Unfortunately, many of the molecules of interest, particularly chemokines and cytokines, are known to be generated during the inflammatory response to wounding and the subsequent cellular events leading to wound repair. With more than 11,000 reports citing the use of microdialysis sampling, only a few researchers have sought to assess the tissue damage that is incurred by probe insertion. For this reason, caution is warranted when collecting these molecules and inferring a role for them in clinical disease states. This commentary seeks to remind the research community of the confounding effects that signaling molecules related to the wounding response will have on clinical studies. Proper controls must be incorporated into all studies in order to assess whether or not particular molecules are truly related to the disease state under investigation or have been generated as part of the tissue response to the wound incurred by microdialysis catheter implantation.





Similar content being viewed by others
Notes
The Stenken group experience with microdialysis sampling probes is that polycarbonate/polyether (PC) membranes were able to function up to 7 days and PES probes to 10 days. However, PC membranes are no longer available and have been replaced with a polyarylethersulfone membrane. We have not tested the long-term implantation viability of these membranes in animals.
References
Westerink BHC, Cremers TIFH, eds. Handbook of microdialysis sampling: methods, applications, and clinical aspects. Amsterdam: Academic; 2007.
Helmy A, Carpenter KLH, Skepper JN, Kirkpatrick PJ, Pickard JD, Hutchinson PJ. Microdialysis of cytokines: methodological considerations, scanning electron microscopy, and determination of relative recovery. J Neurotrauma. 2009;26:549–61.
Waelgaard L, Thorgersen EB, Line P-D, Foss A, Mollnes TE, Tonnessen TI. Microdialysis monitoring of liver grafts by metabolic parameters, cytokine production, and complement activation. Transplantation. 2008;86:1096–103.
Murdolo G, Herder C, Wang Z, Rose B, Schmelz M, Jansson P-A. In situ profiling of adipokines in subcutaneous microdialysates from lean and obese individuals. Am J Physiol. 2008;295:E1095–105.
Mellergard P, Aneman O, Sjogren F, Pettersson P, Hillman J. Changes in extracellular concentrations of some cytokines, chemokines, and neurotrophic factors after insertion of intracerebral microdialysis catheters in neurosurgical patients. Neurosurgery. 2008;62:151–7. Discussion 157–8.
Groth L, Jorgensen A, Serup J. Cutaneous microdialysis in the rat: insertion trauma and effect of anaesthesia studied by laser Doppler perfusion imaging and histamine release. Skin Pharmacol Appl Skin Physiol. 1998;11:125–32.
Mitala CM, Wang Y, Borland LM, Jung M, Shand S, Watkins S, et al. Impact of microdialysis probes on vasculature and dopamine in the rat striatum: a combined fluorescence and voltammetric study. J Neurosci Methods. 2008;174:177–85.
Morgan ME, Singhal D, Anderson BD. Quantitative assessment of blood–brain barrier damage during microdialysis. J Pharmacol Exp Ther. 1996;277:1167–76.
Benveniste H, Diemer NH. Cellular reactions to implantation of a microdialysis tube in the rat hippocampus. Acta Neuropathol. 1987;74:234–8.
Benveniste H, Hansen AJ, Ottosen NS. Determination of brain interstitial concentrations by microdialysis. J Neurochem. 1989;52:1741–50.
Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. J Neurosci Methods. 1999;90:129–42.
Zhou F, Zhu X, Castellani RJ, Stimmelmayr R, Perry G, Smith MA, et al. Hibernation, a model of neuroprotection. Am J Pathol. 2001;158:2145–51.
de Lange ECM, Danhof M, de Boer AG, Breimer DD. Critical factors of intracerebral microdialysis as a technique to determine the pharmacokinetics of drugs in rat brain. Brain Res. 1994;666:1–8.
Peters JL, Miner LH, Michael AC, Sesack SR. Ultrastructure at carbon fiber microelectrode implantation sites after acute voltammetric measurements in the striatum of anesthetized rats. J Neurosci Methods. 2004;137:9–23.
Petersen LJ, Poulsen LK, Sondergaard J, Skov PS. The use of cutaneous microdialysis to measure substance P-induced histamine release in intact human skin in vivo. J Allergy Clin Immunol. 1994;94:773–83.
Clough GF, Jackson CL, Lee JJ, Jamal SC, Church MK. What can microdialysis tell us about the temporal and spatial generation of cytokines in allergen-induced responses in human skin in vivo? J Invest Dermatol. 2007;127:2799–806.
Zweiman B, Kaplan AP, Tong L, Moskovitz AR. Cytokine levels and inflammatory responses in developing late-phase allergic reactions in the skin. J Allergy Clin Immunol. 1997;100:104–9.
Sjogren F, Svensson C, Anderson C. Technical prerequisites for in vivo microdialysis determination of interleukin-6 in human dermis. Br J Dermatol. 2002;146:375–82.
Averbeck M, Beilharz S, Bauer M, Gebhardt C, Hartmann A, Hochleitner K, et al. In situ profiling and quantification of cytokines released during ultraviolet B-induced inflammation by combining dermal microdialysis and protein microarrays. Exp Dermatol. 2006;15:447–54.
Angst MS, Clark JD, Carvalho B, Tingle M, Schmelz M, Yeomans DC. Cytokine profile in human skin in response to experimental inflammation, noxious stimulation, and administration of a COX-inhibitor: a microdialysis study. Pain. 2008;139:15–27.
Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100.
Grabb MC, Sciotti VM, Gidday JM, Cohen SA, Van Wylen DGL. Neurochemical and morphological responses to acutely and chronically implanted brain microdialysis probes. J Neurosci Methods. 1998;82:25–34.
Wisniewski N, Rajamand N, Adamsson U, Lins PE, Reichert WM, Klitzman B, et al. Analyte flux through chronically implanted subcutaneous polyamide membranes differs in humans and rats. Am J Physiol. 2002;282:E1316–23.
Wisniewski N, Klitzman B, Miller B, Reichert WM. Decreased analyte transport through implanted membranes: differentiation of biofouling from tissue effects. J Biomed Mater Res. 2001;57:513–21.
Wientjes KJC, Grob U, Hattemer A, Hoogenberg K, Jungheim K, Kapitza C, et al. Effects of microdialysis catheter insertion into the subcutaneous adipose tissue assessed by the SCGM1 system. Diabetes Technol Ther. 2003;5:615–20.
Wang X, Lennartz MR, Loegering DJ, Stenken JA. Interleukin-6 collection through long-term implanted microdialysis sampling probes in rat subcutaneous space. Anal Chem. 2007;79:1816–24.
Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. J Invest Dermatol. 1994;102:807–11.
Clough GF, Boutsiouki P, Church MK, Michel CC. Effects of blood flow on the in vivo recovery of a small diffusible molecule by microdialysis in human skin. J Pharmacol Exp Ther. 2002;302:681–6.
Benveniste H, Huttemeier PC. Microdialysis—theory and application. Prog Neurobiol. 1990;35:195–215.
Kehr J. A survey on quantitative microdialysis: theoretical models and practical implications. J Neurosci Methods. 1993;48:251–61.
Bungay PM, Morrison PF, Dedrick RL. Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci. 1990;46:105–19.
Bungay PM, Newton-Vinson P, Isele W, Garris PA, Justice JB Jr. Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. J Neurochem. 2003;86:932–46.
Petersen LJ. Quantitative measurement of extracellular histamine concentrations in intact human skin in vivo by the microdialysis technique: methodological aspects. Allergy. 1997;52:547–55.
Sugawara T, Gallucci RM, Simeonova PP, Luster MI. Regulation and role of Interleukin 6 in wounded human epithelial keratinocytes. Cytokine. 2001;15:328–36.
Sehgal PB. Interleukin-6: molecular pathophysiology. J Invest Dermatol. 1990;94:2S–6.
Mou X. Modulation of foreign body response towards implanted microdialysis probes; Ph.D. dissertation, Rensselaer Polytechnic Institute; 2007.
Stenken JA. Membrane-based separations applied to in vivo glucose sensing—microdialysis and ultrafiltration sampling. In: Cunningham DD, Stenken JA, editors. In vivo glucose sensing. Hoboken: Wiley; 2010. p. 157–90.
Acknowledgments
JAS acknowledges NIH EB 001441 for funding. CAG acknowledges the Geraldine Kerkut Studentship for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stenken, J.A., Church, M.K., Gill, C.A. et al. How Minimally Invasive is Microdialysis Sampling? A Cautionary Note for Cytokine Collection in Human Skin and other Clinical Studies. AAPS J 12, 73–78 (2010). https://doi.org/10.1208/s12248-009-9163-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-009-9163-7