Abstract
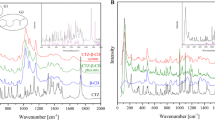
The objective of the present research was to evaluate the physicochemical characteristics of berberine chloride and to assess the complexation of drug with 2-hydroxypropyl-β-cyclodextrin (HPβCD), a first step towards solution dosage form development. The parameters such as log P value were determined experimentally and compared with predicted values. The pH-dependent aqueous solubility and stability were investigated following standard protocols at 25°C and 37°C. Drug solubility enhancement was attempted utilizing both surfactants and cyclodextrins (CDs), and the drug/CD complexation was studied employing various techniques such as differential scanning calorimetry, Fourier transform infrared, nuclear magnetic resonance, and scanning electron microscopy. The experimental log P value suggested that the compound is fairly hydrophilic. Berberine chloride was found to be very stable up to 6 months at all pH and temperature conditions tested. Aqueous solubility of the drug was temperature dependent and exhibited highest solubility of 4.05 ± 0.09 mM in phosphate buffer (pH 7.0) at 25°C, demonstrating the effect of buffer salts on drug solubility. Decreased drug solubility was observed with increasing concentrations of ionic surfactants such as sodium lauryl sulfate and cetyl trimethyl ammonium bromide. Phase solubility studies demonstrated the formation of berberine chloride–HPβCD inclusion complex with 1:1 stoichiometry, and the aqueous solubility of the drug improved almost 4.5-fold in the presence of 20% HPβCD. The complexation efficiency values indicated that the drug has at least threefold greater affinity for hydroxypropyl-β-CD compared to randomly methylated-β-CD. The characterization techniques confirmed inclusion complex formation between berberine chloride and HPβCD and demonstrated the feasibility of developing an oral solution dosage form of the drug.








Similar content being viewed by others
References
Zuo F, Nakamura N, Akao T, Hattori M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab Dispos. 2006;34(12):2064–72.
Dostál J, Man S, Seckárová P, Hulová D, Necas M, Potácek M, et al. Berberine and coptisine free bases. J Mol Struct. 2004;687(1–3):135–42.
Hayashi K, Minoda K, Nagaoka Y, Hayashi T, Uesato S. Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg Med Chem Lett. 2007;17(6):1562–4.
Birdsall TC, Kelly GS. Berberine: therapeutic potential of an alkaloid found in several medicinal plants. Altern Med Rev. 1997;2(2):94–103.
Taylor CT, Winter DC, Skelly MM, O'Donoghue DP, O'Sullivan GC, Harvey BJ, et al. Berberine inhibits ion transport in human colonic epithelia. Eur J Pharmacol. 1999;368(1):111–8.
Tran QL, Tezuka Y, Ueda JY, Nguyen NT, Maruyama Y, Begum K, et al. In vitro antiplasmodial activity of antimalarial medicinal plants used in Vietnamese traditional medicine. J Ethnopharmacol. 2003;86(2–3):249–52.
Ko WH, Yao XQ, Lau CW, Law WI, Chen ZY, Kwok W, et al. Vasorelaxant and antiproliferative effects of berberine. Eur J Pharmacol. 2000;399(2–3):187–96.
Sanchez-Chapula J. Increase in action potential duration and inhibition of the delayed rectifier outward current IK by berberine in cat ventricular myocytes. Br J Pharmacol. 1996;117(7):1427–34.
Tsai PL, Tsai TH. Hepatobiliary excretion of berberine. Drug Metab Dispos. 2004;32(4):405–12.
Pan GY, Huang ZJ, Wang GJ, Fawcett JP, Liu XD, Zhao XC, et al. The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta Med. 2003;69(7):632–6.
Jantova S, Cipak L, Cernakova M, Kost'alova D. Effect of berberine on proliferation, cell cycle and apoptosis in HeLa and L1210 cells. J Pharm Pharmacol. 2003;55(8):1143–9.
Kettmann V, Kosfalova D, Jantova S, Cernakova M, Drimal J. In vitro cytotoxicity of berberine against HeLa and L1210 cancer cell lines. Pharmazie. 2004;59(7):548–51.
Kupeli E, Kosar M, Yesilada E, Husnu K, Baser C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002;72(6):645–57.
Yesilada E, Kupeli E. Berberis crataegina DC. root exhibits potent anti-inflammatory, analgesic and febrifuge effects in mice and rats. J Ethnopharmacol. 2002;79(2):237–48.
Lu Y-C, Lin Q, Luo G-S, Dai Y-Y. Solubility of berberine chloride in various solvents. J Chem Eng Data. 2006;51(2):642–4.
Misik V, Bezakova L, Malekova L, Kostalova D. Lipoxygenase inhibition and antioxidant properties of protoberberine and aporphine alkaloids isolated from Mahonia aquifolium. Planta Med. 1995;61(4):372–3.
Ma L, Xiao P, Guo B, Wu J, Liang F, Dong S. Cerebral protective effects of some compounds isolated from traditional Chinese herbs. Zhongguo Zhong Yao Za Zhi. 1999;24(4):238–9. 56-inside back cover.
Doggrell SA. Berberine−a novel approach to cholesterol lowering. Expert Opin Investig Drugs. 2005;14(5):683–5.
Alan WB, Cormac TT, David JB. Non-antibiotic anti-diarrhoeal drugs: factors affecting oral bioavailability of berberine and loperamide intestinal tissue. Adv Drug Deliv Rev. 1997;23:111–20.
Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY. The involvement of P-glycoprotein in berberine absorption. Pharmacol Toxicol. 2002;91(4):193–7.
Gurley BJ, Swain A, Hubbard MA, Williams DK, Barone G, Hartsfield F, et al. Clinical assessment of CYP2D6-mediated herb-drug interactions in humans: effects of milk thistle, black cohosh, goldenseal, kava kava, St. John's wort, and Echinacea. Mol Nutr Food Res. 2008;52(7):755–63.
Ye M, Fu S, Pi R, He F. Neuropharmacological and pharmacokinetic properties of berberine: a review of recent research. J Pharm Pharmacol. 2009;61(7):831–7.
Hamer DHLGS. Infectious diarrhea and bacterial food poisoning. In: Feldman M, Scharschmidt BF, Sleisenger MH, editors. Sleisinger and Fordtran's gastrointestinaland liver disease. 6th ed. Philadelphia: WB Saunders; 1998. p. 1594–632.
Liebelt EL. Clinical and laboratory evaluation and management of children with vomiting, diarrhea, and dehydration. Curr Opin Pediatr. 1998;10(5):461–9.
Streit JM, Jones RN, Toleman MA, Stratchounski LS, Fritsche TR. Prevalence and antimicrobial susceptibility patterns among gastroenteritis-causing pathogens recovered in Europe and Latin America and Salmonella isolates recovered from bloodstream infections in North America and Latin America: report from the SENTRY Antimicrobial Surveillance Program (2003). Int J Antimicrob Agents. 2006;27(5):367–75.
Battu SK, Repka MA, Majumdar S, Madhusudan RY. Formulation and evaluation of rapidly disintegrating fenoverine tablets: effect of superdisintegrants. Drug Dev Ind Pharm. 2007;33(11):1225–32.
Patel NA, Patel NJ, Patel RP, Patel RK. The formulation and evaluation of topical berberine hydrochloride products. Pharm Technol. 2010;34:60–9.
Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212.
Koide T, Iwata M, Maekawa K, Saito H, Tanimoto T, Okada S. [Berberine Hydrochloride Reference Standard (Control 001) of National Institute of Health Sciences]. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2001(119):97–100.
Tripathi AN, Chauhan L, Thankachan PP, Barthwal R. Quantum chemical and nuclear magnetic resonance spectral studies on molecular properties and electronic structure of berberine and berberrubine. Magn Reson Chem. 2007;45(8):647–55.
Avdeef A. Physicochemical profiling (solubility, permeability and charge state). Curr Top Med Chem. 2001;1(4):277–351.
Fernandes J, Gattass CR. Topological polar surface area defines substrate transport by multidrug resistance associated protein 1 (MRP1/ABCC1). J Med Chem. 2009;52(4):1214–8.
Hancock BC, York P, Rowe RC. The use of solubility parameters in pharmaceutical dosage form design. Int J Pharm. 1997;148:1–21. Invited Review.
Mannhold R, Poda GI, Ostermann C, Tetko IV. Calculation of molecular lipophilicity: state-of-the-art and comparison of log P methods on more than 96,000 compounds. J Pharm Sci. 2009;98(3):861–93.
Jain A, Ran Y, Yalkowsky SH. Effect of pH-sodium lauryl sulfate combination on solubilization of PG-300995 (an anti-HIV agent): a technical note. AAPS PharmSciTech. 2004;5(3):e45.
Rangel-Yagui CO, Hsu HWL, Pessoa Jr A, Tavares LC. Micellar solubilization of ibuprofen—influence of surfactant head groups on the extent of solubilization. Braz J Pharm Sci. 2005;41(2):237–46.
Duchene D, Glomot FCV. Cyclodextrins and their industrial uses. In: Duchene D, editor. Pharmaceutical applications of cyclodextrins. 1st ed. France: Paris; 1987.
Masson M, Loftsson T, Masson G, Stefansson E. Cyclodextrins as permeation enhancers: some theoretical evaluations and in vitro testing. J Control Release. 1999;59(1):107–18.
Wagh VD, Ghadlinge SV. Taste masking methods and techniques in oral pharmaceuticals: current perspectives. J Pharm Res. 2009;2(6):1049–54.
Patel AR, Vavia PR. Preparation and evaluation of taste masked famotidine formulation using drug/beta-cyclodextrin/polymer ternary complexation approach. AAPS PharmSciTech. 2008;9(2):544–50.
Nagase Y, Hirata M, Wada K, Arima H, Hirayama F, Irie T, et al. Improvement of some pharmaceutical properties of DY-9760e by sulfobutyl ether beta-cyclodextrin. Int J Pharm. 2001;229(1–2):163–72.
Loftsson T, Hreinsdottir D, Masson M. Evaluation of cyclodextrin solubilization of drugs. Int J Pharm. 2005;302(1–2):18–28.
Sharma RK, Wooten JB, Baliga VL, Lin X, Chan WG, Hajaligol MR. Characterization of chars from pyrolysis of lignin. Fuel. 2004;83(11–12):1469–82.
Page PCB, Barros D, Buckley BR, Marples BA. Organocatalysis of asymmetric epoxidation mediated by iminium salts: comments on the mechanism. Tetrahedron: Assymetry. 2005;16(21):3488–91.
Juan Hikawczuk VE, Rossomando PC, Giordano OS, Saad JR. neo-Clerodane diterpenoids from Baccharis flabellata. Phytochemistry. 2002;61(4):389–94.
Miller LA, Carrier RL, Ahmed I. Practical considerations in development of solid dosage forms that contain cyclodextrin. J Pharm Sci. 2007;96(7):1691–707.
Acknowledgements
This project was supported by Grant No. 5P20RR021929 from the National Institute of Health/National Center for Research Resources (NIH/NCRR) and the Grant No. D1 BIT 16663 (DHHS/HRSA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Battu, S.K., Repka, M.A., Maddineni, S. et al. Physicochemical Characterization of Berberine Chloride: A Perspective in the Development of a Solution Dosage Form for Oral Delivery. AAPS PharmSciTech 11, 1466–1475 (2010). https://doi.org/10.1208/s12249-010-9520-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-010-9520-y