Abstract
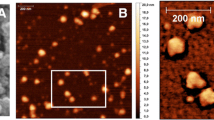
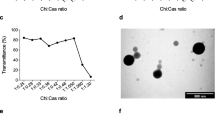
The purpose of this research was to prepare poly-(ε-caprolactone) (PCL) particles by an emulsion-diffusion-evaporation method using a blend of poly-(vinyl alcohol) and chitosan derivatives as stabilizers. The chitosan derivatives used were chitosan hydrochloride and trimethyl chitosans (TMC) with varying degrees of quaternization. Particle characteristics-size, zeta potential, surface morphology, cytotoxicity, and transfection efficiency-were investigated. The developed method yields PCL nanoparticles in the size range of 250 to 300 nm with a positive surface charge (2.5 to 6.8 mV). The cytotoxicity was found to be moderate and virtually independent of the stabilizers' concentration with the exception of the highly quaternized TMC (degree of substitution 66%) being significantly more toxic. In immobilization experiments with gel electrophoresis, it could be shown that these cationic nanoparticles (NP) form stable complexes with DNA at a NP:DNA ratio of 3:1. These nanoplexes showed a significantly higher transfection efficiency on COS-1 cells than naked DNA.
Similar content being viewed by others
References
Crystal RG. The gene as drug.Nat Med. 1995;1:15–17.
Florence AT, Sakthivel T, Toth I. Orral uptake and translocation of a polylysine dendrimer with a lipid surface.J Control Release. 2000;65:253–259.
Ramaswamy C, Sakthivel T, Wilderspin AF, Florence AT. Dendriplexes and their characterization.Int J Pharm. 2003;254:17–21.
Pouton CW, Lucas P, Thomas BJ, Uduehi AN, Milroy DA, Moss SH. Polycation-DNA complexes for gene delivery: a comparison of the biopharmaceutical properties of cationic polypeptides and cationic lipids.J Control Release. 1998;53:289–299.
Ramsay E, Gumbleton M. Polylysine and polyornithine gene transfer complexes: a study of complex stability and cellular uptake as a basis for their differential in-vitro transfection efficiency.J Drug Target. 2002;10:1–9.
Oupicky D, Konak C, Ulbrich K, Wolfert MA, Seymour LW. DNA delivery systems based on complexes of DNA with synthetic polycations and their copolymers.J Control Release. 2000;65:149–171.
Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicle.Proc Natl Acad Sci U S A. 1992;89:7934–7938.
Fischer D, Bieber T, Li Y, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity.Pharm Res. 1999;16:1273–1279.
Kunath K, von Harpe A, Fischer D, et al. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine.J Control Release. 2003;89:113–125.
Smith JG, Wedeking T, Vernachio JH, Way H, Niven RW. Characterization and in vivo testing of a heterogeneous cationic lipid-DNA formulation.Pharm Res. 1998;15:1356–1363.
Olbrich C, Bakowsky U, Lehr CM, Muller RH, Kneuer C. Cationic solid-lipid nanoparticles can efficiently bind and transfect plasmid DNA.J Control Release. 2001;77:345–355.
Oberle V, Bakowsky U, Zuhorn IS, Hoekstra D. Lipoplex formation under equilibrium conditions reveals a three step mechanism.Biophys J. 2000;79:1447–1454.
Mahato RI, Kawabata K, Nomura T, Takakura Y, Hashida M. Physicochemical and pharmacokinetic characteristics of plasmid DNA/cationic liposome complexes.J Pharm Sci. 1995;84:1267–1271.
Farhood H, Serbina N, Huang L. The role of dioleyl phosphatidylethanolamine in cationic liposome mediated gene transfer.Biochim Biophys Acta. 1995;1235:289–295.
Sternberg B, Hong K, Zheng W, Papahadjopoulos D. Ultrastructural characterization of cationic liposome-DNA complexes showing enhanced stability in serum and high transfection activity in vivo.Biochim Biophys Acta. 1998;1375;23–35.
Meyer O, Kirpotin D, Hong K, Sternberg B, Park JW, Woodle MC, Papahadjopoulos D. Cationic liposomes coated with polyethylene glycol as carriers for oligonucleotides.J Biol Chem. 1998;273:15621–15627.
Behr JP, Demeneix B, Loeffler JP, Perez-Mutul J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyaminecoated DNA.Proc Natl Acad Sci U S A. 1989;86:6982–6986.
Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D'Souza GG. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes.Proc Natl Acad Sci U S A. 2003;100:1972–1977.
Cui Z, Mumper RJ. Plasmid DNA-entrapped nanoparticles engineered from microemulsion precursors: in vitro and in vivo evaluation.Bioconjug Chem. 2002;13:1319–1327.
Kneuer C, Sameti M, Haltner EG, Schiestel T, Schirra H, Schmidt H, Lehr CM. Silica nanoparticles modified with aminosilanes as carriers for plasmid DNA.Int J Pharm. 2000;196:257–261.
Kneuer C, Sameti M, Bakowsky U, et al. A nonviral DNA delivery system based on surface modified silica-nanoparticles can efficiently transfect cells in vitro.Bioconjug Chem. 2000;11:926–932.
Ravi Kumar MNV, Bakowsky U, Lehr C-M. Preparation and characterization of cationic PLGA nanospheres as DNA carriers.Biomaterials. 2004;25:1771–1777.
Ravi Kumar MNV, Mohapatra SS, Kong X, Jena PK, Bakowsky ULehr C-M. Cationic poly(lactide-co-glycolide) nanoparticles as efficient in vivo gene transfection agents.J Nanosci Nanotechnol. 2004;4:990–994.
Leong KW, Mao HQ, Truong Le VL, Roy K, Walsh SM, August JT. DNA-polycation nanospheres as non-viral gene delivery vehicles.J Control Release. 1998;53:183–193.
Capan Y, Woo BH, Gebrekidan S, Ahmed S, DeLuca PP. Preparation and characterization of poly (D,L-lactide-co-glycolide) microspheres for controlled release of poly(L-lysine) complexed plasmid DNA.Pharm Res. 1999;16:509–513.
Ravi Kumar MNV, Sameti M, Mohapatra SS, Kong X, Lockey RF, Bakowsky U, Lindenblatt G, Schmidt H, Lehr CM. Cationic silica nanoparticles as gene carriers: synthesis, characterization and transfection efficiency in vitro in vivo.J Nanosci Nanotechnol. 2004;4:876–881.
Florea BI, Meaney C, Junginger HE, Borchard G. Transfection efficiency and toxicity of polyethylenimine in differentiated Calu-3 and nondifferentiated COS-1 cell cultures.AAPS PharmSci. 2003;4:E12.
Merdan T, Kopecek J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer.Adv Drug Deliv Rev. 2002;54:715–758.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: August 10, 2005
Rights and permissions
About this article
Cite this article
Haas, J., Ravi Kumar, M.N.V., Borchard, G. et al. Preparation and characterization of chitosan and trimethyl-chitosanmodified poly-(ε-caprolactone) nanoparticles as DNA carriers. AAPS PharmSciTech 6, 6 (2005). https://doi.org/10.1208/pt060106
Received:
Accepted:
DOI: https://doi.org/10.1208/pt060106