Abstract
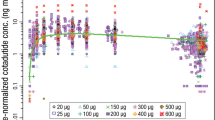
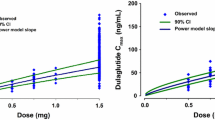
Taranabant is a cannabinoid-1 receptor inverse agonist developed for the treatment of obesity. A population model was constructed to facilitate the estimation of pharmacokinetic parameters and to identify the influence of selected covariates. Data from 12 phase 1 studies and one phase 2 study were pooled from subjects administered single and multiple oral doses of taranabant ranging from 0.5 to 8 mg. A total of 6,834 taranabant plasma concentrations from 187 healthy and 385 obese subjects were used to develop the population model in NONMEM. A standard covariate analysis using forward selection (α = 0.05) and backward elimination (α = 0.001) was conducted. A three-compartment model with first-order absorption and elimination adequately described plasma taranabant concentrations. The population mean estimates for apparent clearance and apparent steady-state volume of distribution were 25.4 L/h and 2,578 L, respectively. Statistically significant covariate effects were modest in magnitude and not considered clinically relevant (the effects of body mass index (BMI) and creatinine clearance (CrCL) on apparent clearance; BMI, age, CrCL, and gender on apparent volume of the peripheral compartment and age on apparent intercompartmental clearance). The pharmacokinetic profile of taranabant can adequately be described by a three-compartment model with first-order absorption and elimination. Clinical dose adjustment based on covariates effects is not warranted.




Similar content being viewed by others
References
Fong TM, Guan XM, Marsh DJ, Shen CP, Stribling DS, Rosko KN, et al. Antiobesity efficacy of a novel cannabinoid-1 receptor inverse agonist, N-[(1 S, 2 S)-3-(4-Chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-{[5-trifluoromethyl)pyridine-2-yl]-oxy}propanamide (MK-0364), in rodents. J Pharmacol Exp Ther. 2007;321(3):1013–22.
Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, et al. The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab. 2008;7(1):68–78.
Gantz I, Erondu N, Suryawanshi S, Musser B, Nayee J, Johnson A, et al. A two-year study to assess the efficacy, safety, and tolerability of taranabant in obese patients: 52 week results. Proceedings of the American College of Cardiology, 57th Annual Scientific Session; Chicago; Illinois; 2008
Addy C, Li S, Agrawak N, Stone J, Majumdar A, Zhong L, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamic properties of taranabant, a novel selective cannabinoid-1 receptor inverse agonist, for the treatment of obesity: results from a double-blind, placebo-controlled, single oral dose study in healthy volunteers. J Clin Pharmacol. 2008;48:418–27.
Addy C, Rothenberg P, Li S, Majumdar A, Agrawal N, Li H, et al. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of taranabant, a novel selective cannabinoid-1 receptor inverse agonist, in healthy male volunteers. J Clin Pharmacol. 2008;48:734–44.
SAS Institute Inc. SAS [computer program]. Version 9.1.3. Cary, NC: SAS Institute. 2006.
S-PLUS 7 for Windows User's Guide. Seattle, WA: Insightful Corporation. 2005.
ICON Development Solutions. NONMEM [computer program]. Version VI. Ellicott City, MD. 2006.
Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82:17–20.
Karlsson MO, Beal SL, Sheiner LB. Three new residual error models for population PK/PD analyses. J Pharmacokinet Biopharm. 1995;23(6):651–72.
Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39(3):215–31.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Nielsen, J., Cirincione, B. et al. Development of a Population Pharmacokinetic Model for Taranabant, a Cannibinoid-1 Receptor Inverse Agonist. AAPS J 12, 537–547 (2010). https://doi.org/10.1208/s12248-010-9212-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-010-9212-2