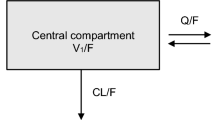
Abstract
A number of classical pharmacokinetic studies have been conducted in transplant patients. However, they suffer from some limitations, for example, (1) the study design was limited to intense blood sampling in small groups of patients during a certain posttransplant period, (2) patient factors were evaluated one at a time to identify their association with the pharmacokinetic parameters, and (3) mean pharmacokinetic parameters often cannot be precisely estimated due to large intraindividual variability. Population pharmacokinetics provides a potential means of addressing these limitations and is a powerful tool to evaluate the magnitude and consistency of drug exposure. Population pharmacokinetic studies of cyclosporine focused solely on developing limited sampling strategies and Bayesian estimators to estimate drug exposure, have been summarized before, and are, therefore, not a subject of this review. The major focus of this review is to describe factors (demographic factors, hepatic and gastrointestinal functions, drug–drug interactions, genetic polymorphisms of drug metabolizing enzymes and transporters) that have been identified to contribute to the large portion of observed variability in the pharmacokinetics of cyclosporine in transplant patients. This review summarizes and interprets the conclusions as well as the nonlinear mixed-effects modeling methodologies used in such studies. A highly diversified collection of structural models, variability models, and covariate submodels have been evaluated and validated using internal or external validation methods. This review also highlights areas where additional research is warranted to improve the models since a portion of model variability still remains unexplained.


Similar content being viewed by others
References
Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetics parameters. I. Michaelis-Menten model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm. 1980;8(6):553–71. PubMed PMID: 7229908. eng.
Sheiner BL, Beal SL. Evaluation of methods for estimating population pharmacokinetic parameters. II. Biexponential model and experimental pharmacokinetic data. J Pharmacokinet Biopharm. 1981;9(5):635–51. PubMed PMID: 7334463. eng.
Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetic parameters. III. Monoexponential model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm. 1983;11(3):303–19. PubMed PMID: 6644555. eng.
Sheiner LB. The population approach to pharmacokinetic data analysis: rationale and standard data analysis methods. Drug Metab Rev. 1984;15(1–2):153–71. PubMed PMID: 6745080. eng.
Sheiner LB, Steimer JL. Pharmacokinetic/pharmacodynamic modeling in drug development. Annu Rev Pharmacol Toxicol. 2000;40:67–95. PubMed PMID: 10836128. eng.
Thomson AH, Whiting B. Bayesian parameter estimation and population pharmacokinetics. Clin Pharmacokinet. 1992;22(6):447–67. PubMed PMID: 1587057. eng.
Ette EI, Williams PJ. Population pharmacokinetics II: estimation methods. Ann Pharmacother. 2004;38(11):1907–15. PubMed PMID: 15367729. eng.
Ette EI, Williams PJ. Population pharmacokinetics I: background, concepts, and models. Ann Pharmacother. 2004;38(10):1702–6. PubMed PMID: 15328391. eng.
Bonate PL. Pharmacokinetic-pharmacodynamic modeling and simulation. New York: Springer; 2006.
Lee TC, Charles B, Steer P, Flenady V, Shearman A. Population pharmacokinetics of intravenous caffeine in neonates with apnea of prematurity. Clin Pharmacol Ther. 1997;61(6):628–40. PubMed PMID: 9209245. Epub 1997/06/01. eng.
McLachlan AJ, Tett SE. Pharmacokinetics of fluconazole in people with HIV infection: a population analysis. Br J Clin Pharmacol. 1996;41(4):291–8. PubMed PMID: 8730974. PubMed Central PMCID: 2042597. Epub 1996/04/01. eng.
Novartis Pharmaceuticals. SANDIMMUNE(R) oral soft gelatin capsules, oral solution, injection for infusion. http://www.pharma.us.novartis.com/cs/www.pharma.us.novartis.com/product/pi/pdf/sandimmune.pdf. 2005.
Novartis Pharmaceuticals. NEORAL(R) soft gelatin capsules, oral solution. http://www.pharma.us.novartis.com/cs/www.pharma.us.novartis.com/product/pi/pdf/neoral.pdf. 2005
Jorga A, Holt DW, Johnston A. Therapeutic drug monitoring of cyclosporine. Transplant Proc. 2004;36(2 Suppl):396S–403S. PubMed PMID: 15041374. eng.
Dunn CJ, Wagstaff AJ, Perry CM, Plosker GL, Goa KL. Cyclosporin: an updated review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation (neoral)1 in organ transplantation. Drugs. 2001;61(13):1957–2016. PubMed PMID: 11708766. eng.
Olyaei AJ, de Mattos AM, Bennett WM. Nephrotoxicity of immunosuppressive drugs: new insight and preventive strategies. Curr Opin Crit Care. 2001;7(6):384–9. PubMed PMID: 11805539. eng.
Mentre F, Mallet A, Steimer JL, Lokiec F. An application of population pharmacokinetics to the clinical use of cyclosporine in bone marrow transplant patients. Transplant Proc. 1988;20(2 Suppl 2):466–70. PubMed PMID: 3284091. eng.
Mallet A, Mentre F, Steimer JL, Lokiec F. Nonparametric maximum likelihood estimation for population pharmacokinetics, with application to cyclosporine. J Pharmacokinet Biopharm. 1988;16(3):311–27. PubMed PMID: 3065480. eng.
Serre-Debeauvais F, Iliadis A, Tranchand B, Michallet M, Benzekri S, Ardiet C, et al. Bayesian estimation of cyclosporine clearance in bone marrow graft. Ther Drug Monit. 1990;12(1):16–22. PubMed PMID: 2305415. eng.
Grevel J, Post BK, Kahan BD. Michaelis-Menten kinetics determine cyclosporine steady-state concentrations: a population analysis in kidney transplant patients. Clin Pharmacol Ther. 1993;53(6):651–60. PubMed PMID: 8513657. eng.
Rui JZ, Zhuo HT, Jiang GH, Chen G. [Evaluation of population pharmacokinetics of cyclosporin A in renal transplantation patients with NONMEM]. Yao Xue Xue Bao. 1995;30(4):241–7. PubMed PMID: 7660791. chi.
Charpiat B, Falconi I, Breant V, Jelliffe RW, Sab JM, Ducerf C, et al. A population pharmacokinetic model of cyclosporine in the early postoperative phase in patients with liver transplants, and its predictive performance with Bayesian fitting. Ther Drug Monit. 1998;20(2):158–64. PubMed PMID: 9558129. eng.
Parke J, Charles BG. NONMEM population pharmacokinetic modeling of orally administered cyclosporine from routine drug monitoring data after heart transplantation. Ther Drug Monit. 1998;20(3):284–93. PubMed PMID: 9631925. eng.
McLachlan AJ, Tett SE. Effect of metabolic inhibitors on cyclosporine pharmacokinetics using a population approach. Ther Drug Monit. 1998;20(4):390–5. PubMed PMID: 9712463. eng.
Kyhl LE, Rasmussen SN, Aarons L, Jensen SB. Population pharmacokinetics of cyclosporine: influence of covariables and assessment of cyclosporine absorption in kidney, lung, heart and heart + lung transplanted patients. Transplant Proc. 1998;30(5):1680. PubMed PMID: 9723241. eng.
Porta B, Perez-Ruixo JJ, Gorriz JL, Crespo JF, Sancho A, Pallardo LM, et al. Population pharmacokinetics of cyclosporine in kidney transplant patients. Transplant Proc. 1999;31(6):2246–7. PubMed PMID: 10500561. eng.
Parke J, Charles BG. Factors affecting oral cyclosporin disposition after heart transplantation: bootstrap validation of a population pharmacokinetic model. Eur J Clin Pharmacol. 2000;56(6–7):481–7. PubMed PMID: 11049011. eng.
Yoshida K, Kimura T, Hamada Y, Saito T, Endo T, Baba S, et al. Comparative study of population pharmacokinetics upon switching of cyclosporine formulation from Sandimmune to Neoral in stable renal transplant patients. Transplant Proc. 2001;33(7-8):3146–7. PubMed PMID: 11750351. eng.
Schadeli F, Marti HP, Frey FJ, Uehlinger DE. Population pharmacokinetic model to predict steady-state exposure to once-daily cyclosporin microemulsion in renal transplant recipients. Clin Pharmacokinet. 2002;41(1):59–69. PubMed PMID: 11825097. eng.
Leger F, Debord J, Le Meur Y, Rousseau A, Buchler M, Lachatre G, et al. Maximum a posteriori Bayesian estimation of oral cyclosporin pharmacokinetics in patients with stable renal transplants. Clin Pharmacokinet. 2002;41(1):71–80. PubMed PMID: 11825098. eng.
Rousseau A, Monchaud C, Debord J, Vervier I, Estenne M, Thiry P, et al. Bayesian forecasting of oral cyclosporin pharmacokinetics in stable lung transplant recipients with and without cystic fibrosis. Ther Drug Monit. 2003;25(1):28–35. PubMed PMID: 12548141. eng.
Jacobson PA, Ng J, Green KG, Rogosheske J, Brundage R. Posttransplant day significantly influences pharmacokinetics of cyclosporine after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2003;9(5):304–11. PubMed PMID: 12766880. eng.
Rousseau A, Leger F, Le Meur Y, Saint-Marcoux F, Paintaud G, Buchler M, et al. Population pharmacokinetic modeling of oral cyclosporin using NONMEM: comparison of absorption pharmacokinetic models and design of a Bayesian estimator. Ther Drug Monit. 2004;26(1):23–30. PubMed PMID: 14749545. eng.
Rousseau A, Marquet P. Modelling ciclosporin double-peak absorption profiles in the early post-transplantation period. Clin Pharmacokinet. 2004;43(14):1055–7. PubMed PMID: 15530133. eng.
Hesselink DA, van Gelder T, van Schaik RH, Balk AH, van der Heiden IP, van Dam T, et al. Population pharmacokinetics of cyclosporine in kidney and heart transplant recipients and the influence of ethnicity and genetic polymorphisms in the MDR-1, CYP3A4, and CYP3A5 genes. Clin Pharmacol Ther. 2004;76(6):545–56. PubMed PMID: 15592326. eng.
Bourgoin H, Paintaud G, Buchler M, Lebranchu Y, Autret-Leca E, Mentre F, et al. Bayesian estimation of cyclosporin exposure for routine therapeutic drug monitoring in kidney transplant patients. Br J Clin Pharmacol. 2005;59(1):18–27. PubMed PMID: 15606436. eng.
Rosenbaum SE, Baheti G, Trull AK, Akhlaghi F. Population pharmacokinetics of cyclosporine in cardiopulmonary transplant recipients. Ther Drug Monit. 2005;27(2):116–22. PubMed PMID: 15795639. eng.
Wu KH, Cui YM, Guo JF, Zhou Y, Zhai SD, Cui FD, et al. Population pharmacokinetics of cyclosporine in clinical renal transplant patients. Drug Metab Dispos. 2005;33(9):1268–75. PubMed PMID: 15932953. eng.
Fradette C, Lavigne J, Waters D, Ducharme MP. The utility of the population approach applied to bioequivalence in patients: comparison of 2 formulations of cyclosporine. Ther Drug Monit. 2005;27(5):592–600. PubMed PMID: 16175132. eng.
Lukas JC, Suarez AM, Valverde MP, Calvo MV, Lanao JM, Calvo R, et al. Time-dependent pharmacokinetics of cyclosporine (Neoral) in de novo renal transplant patients. J Clin Pharm Ther. 2005;30(6):549–57. PubMed PMID: 16336287. eng.
Yin OQ, Lau SK, Chow MS. Population pharmacokinetics of cyclosporine in Chinese cardiac transplant recipients. Pharmacotherapy. 2006;26(6):790–7. PubMed PMID: 16716132. eng.
Saint-Marcoux F, Marquet P, Jacqz-Aigrain E, Bernard N, Thiry P, Le Meur Y, et al. Patient characteristics influencing ciclosporin pharmacokinetics and accurate Bayesian estimation of ciclosporin exposure in heart, lung and kidney transplant patients. Clin Pharmacokinet. 2006;45(9):905–22. PubMed PMID: 16928152. eng.
Irtan S, Saint-Marcoux F, Rousseau A, Zhang D, Leroy V, Marquet P, et al. Population pharmacokinetics and bayesian estimator of cyclosporine in pediatric renal transplant patients. Ther Drug Monit. 2007;29(1):96–102. PubMed PMID: 17304156. eng.
Fanta S, Jonsson S, Backman JT, Karlsson MO, Hoppu K. Developmental pharmacokinetics of ciclosporin—a population pharmacokinetic study in paediatric renal transplant candidates. Br J Clin Pharmacol. 2007;64(6):772–84. PubMed PMID: 17662086. eng.
Fanta S, Niemi M, Jonsson S, Karlsson MO, Holmberg C, Neuvonen PJ, et al. Pharmacogenetics of cyclosporine in children suggests an age-dependent influence of ABCB1 polymorphisms. Pharmacogenet Genomics. 2008;18(2):77–90. PubMed PMID: 18192894. eng.
Willemze AJ, Cremers SC, Schoemaker RC, Lankester AC, den Hartigh J, Burggraaf J, et al. Ciclosporin kinetics in children after stem cell transplantation. Br J Clin Pharmacol. 2008;66(4):539–45. PubMed PMID: 18492124. eng.
Falck P, Midtvedt K, Van Le TT, Storehagen L, Holdaas H, Hartmann A, et al. A population pharmacokinetic model of ciclosporin applicable for assisting dose management of kidney transplant recipients. Clin Pharmacokinet. 2009;48(9):615–23. PubMed PMID: 19725595. eng.
Chen B, Zhang W, Gu Z, Li J, Zhang Y, Cai W. Population pharmacokinetic study of cyclosporine in Chinese renal transplant recipients. Eur J Clin Pharmacol. 2011;67(6):601–12. PubMed PMID: 21161198. Epub 2010/12/17. eng.
Eljebari H, Gaies E, Fradj NB, Jebabli N, Salouage I, Trabelsi S, et al. Population pharmacokinetics and Bayesian estimation of cyclosporine in a Tunisian population of hematopoietic stem cell transplant recipient. Eur J Clin Pharmacol. 2012;68(11):1517–24. PubMed PMID: 22527344. Epub 2012/04/25. eng.
Ji E, Kim MY, Yun HY, Kim KI, Kang W, Kwon KI, et al. Population pharmacokinetics of cyclosporine in Korean adults undergoing living-donor kidney transplantation. Pharmacotherapy. 2011;31(6):574–84. PubMed PMID: 21923441. Epub 2011/09/20. eng.
Song J, Kim MG, Choi B, Han NY, Yun HY, Yoon JH, et al. CYP3A5 polymorphism effect on cyclosporine pharmacokinetics in living donor renal transplant recipients: analysis by population pharmacokinetics. Ann Pharmacother. 2012;46(9):1141–51. PubMed PMID: 22947591. Epub 2012/09/06. eng.
Sun B, Li XY, Gao JW, Rui JZ, Guo YK, Peng ZH, et al. Population pharmacokinetic study of cyclosporine based on NONMEM in Chinese liver transplant recipients. Ther Drug Monit. 2010;32(6):715–22. PubMed PMID: 21068646. Epub 2010/11/12. eng.
Wilhelm AJ, de Graaf P, Veldkamp AI, Janssen JJ, Huijgens PC, Swart EL. Population pharmacokinetics of ciclosporin in haematopoietic allogeneic stem cell transplantation with emphasis on limited sampling strategy. Br J Clin Pharmacol. 2012;73(4):553–63. PubMed PMID: 21988410. PubMed Central PMCID: PMC3376432. Epub 2011/10/13. eng.
Zhou H, Gao Y, Cheng XL, Li ZD. Population pharmacokinetics of cyclosporine A based on NONMEM in Chinese allogeneic hematopoietic stem cell transplantation recipients. Eur J Drug Metab Pharmacokinet. 2012;37(4):271–8. PubMed PMID: 22446981. Epub 2012/03/27. eng.
Debord J, Risco E, Harel M, Le Meur Y, Buchler M, Lachatre G, et al. Application of a gamma model of absorption to oral cyclosporin. Clin Pharmacokinet. 2001;40(5):375–82. PubMed PMID: 11432538. eng.
Krejcie TC, Jacquez JA, Avram MJ, Niemann CU, Shanks CA, Henthorn TK. Use of parallel Erlang density functions to analyze first-pass pulmonary uptake of multiple indicators in dogs. J Pharmacokinet Biopharm. 1996;24(6):569–88. PubMed PMID: 9300351. eng.
Niemann CU, Henthorn TK, Krejcie TC, Shanks CA, Enders-Klein C, Avram MJ. Indocyanine green kinetics characterize blood volume and flow distribution and their alteration by propranolol. Clin Pharmacol Ther. 2000;67(4):342–50. PubMed PMID: 10801242. eng.
Premaud A, Debord J, Rousseau A, Le Meur Y, Toupance O, Lebranchu Y, et al. A double absorption-phase model adequately describes mycophenolic acid plasma profiles in de novo renal transplant recipients given oral mycophenolate mofetil. Clin Pharmacokinet. 2005;44(8):837–47. PubMed PMID: 16029068. eng.
Christenson JT, Schmuziger M, Maurice J, Simonet F, Velebit V. Postoperative visceral hypotension the common cause for gastrointestinal complications after cardiac surgery. Thorac Cardiovasc Surg. 1994;42(3):152–7. PubMed PMID: 7940485. eng.
Kennedy JM, Riji AM. Effects of surgery on the pharmacokinetic parameters of drugs. Clin Pharmacokinet. 1998;35(4):293–312. PubMed PMID: 9812179. eng.
Vantrappen G, Janssens J, Peeters TL, Bloom SR, Christofides ND, Hellemans J. Motilin and the interdigestive migrating motor complex in man. Dig Dis Sci. 1979;24(7):497–500. PubMed PMID: 456236. eng.
Drewe J, Beglinger C, Kissel T. The absorption site of cyclosporin in the human gastrointestinal tract. Br J Clin Pharmacol. 1992;33(1):39–43. PubMed PMID: 1540489. eng.
Ptachcinski RJ, Venkataramanan R, Burckart GJ. Clinical pharmacokinetics of cyclosporin. Clin Pharmacokinet. 1986;11(2):107–32. PubMed PMID: 3514043. eng.
Shaw L, Bowers L, Demers L. Critical issues in cyclosporine monitoring: report of the Task Force on Cyclosporine Monitoring. Clin Chem. 1987;33(7):1269–88. PubMed PMID: 3297427. eng.
Lindholm A. Factors influencing the pharmacokinetics of cyclosporine in man. Ther Drug Monit. 1991;13(6):465–77. PubMed PMID: 1771643. eng.
Bowers LD, Canafax DM. Cyclosporine: experience with therapeutic monitoring. Ther Drug Monit. 1984;6(2):142–7. PubMed PMID: 6377597. eng.
Rowland M, Gupta SK. Cyclosporin-phenytoin interaction: re-evaluation using metabolite data. Br J Clin Pharmacol. 1987;24(3):329–34. PubMed PMID: 3663449. eng.
Hebert MF, Roberts JP, Prueksaritanont T, Benet LZ. Bioavailability of cyclosporine with concomitant rifampin administration is markedly less than predicted by hepatic enzyme induction. Clin Pharmacol Ther. 1992;52(5):453–7. PubMed PMID: 1424418. eng.
Wu CY, Benet LZ, Hebert MF, Gupta SK, Rowland M, Gomez DY, et al. Differentiation of absorption and first-pass gut and hepatic metabolism in humans: studies with cyclosporine. Clin Pharmacol Ther. 1995;58(5):492–7. PubMed PMID: 7586942. eng.
Yee GC, McGuire TR, Gmur DJ, Lennon TP, Deeg HJ. Blood cyclosporine pharmacokinetics in patients undergoing marrow transplantation. Influence of age, obesity, and hematocrit. Transplantation. 1988;46(3):399–402. PubMed PMID: 3047931. eng.
Lindholm A, Welsh M, Alton C, Kahan BD. Demographic factors influencing cyclosporine pharmacokinetic parameters in patients with uremia: racial differences in bioavailability. Clin Pharmacol Ther. 1992;52(4):359–71. PubMed PMID: 1330397. eng.
Venkataramanan R, Burchart G, Ptachcinski RJ. Cyclosporine pharmacokinetics in heart transplant patients. Transplant Proc. 1986;18:768–70. PubMed PMID: 8787947. eng.
Knoop C, Vervier I, Thiry P, De Backer M, Kovarik JM, Rousseau A, et al. Cyclosporine pharmacokinetics and dose monitoring after lung transplantation: comparison between cystic fibrosis and other conditions. Transplantation. 2003;76(4):683–8. PubMed PMID: 12973109. eng.
Canafax DM, Cippole R, Hrushesky W. The chronopharmaceutics of cyclosporine and its metabolites in recipients of pancreas allografts. Transplant Proc. 1998;20(2):471–7.
Analytical Services International Ltd. International Cyclosporin Proficiency Testing Scheme. http://www.bioanalytics.co.uk (accessed December 2009).
Aweeka FT, Tomlanovich SJ, Prueksaritanont T, Gupta SK, Benet LZ. Pharmacokinetics of orally and intravenously administered cyclosporine in pre-kidney transplant patients. J Clin Pharmacol. 1994;34(1):60–7. PubMed PMID: 8132853. eng.
Schwinghammer TL, Przepiorka D, Venkataramanan R, Wang CP, Burckart GJ, Rosenfeld CS, et al. The kinetics of cyclosporine and its metabolites in bone marrow transplant patients. Br J Clin Pharmacol. 1991;32(3):323–8. PubMed PMID: 1777368. PubMed Central PMCID: 1368525. Epub 1991/09/01. eng.
Kivisto KT. A review of assay methods for cyclosporin. Clinical implications. Clin Pharmacokinet. 1992;23(3):173–90. PubMed PMID: 1511535. eng.
Safarcik K, Brozmanova H, Bartos V, Jegorov A, Grundmann M. Evaluation and comparison of therapeutic monitoring of whole-blood levels of cyclosporin A and its metabolites in renal transplantation by HPLC and RIA methods. Clin Chim Acta. 2001;310(2):165–71. PubMed PMID: 11498082. eng.
Conflict of Interest
The authors have no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, K., Pillai, V.C. & Venkataramanan, R. Population Pharmacokinetics of Cyclosporine in Transplant Recipients. AAPS J 15, 901–912 (2013). https://doi.org/10.1208/s12248-013-9500-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-013-9500-8