Abstract
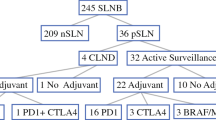
The Sunbelt Melanoma Trial, a multicenter, prospective randomized clinical study, evaluated the role of high-dose interferon alfa-2b (HDI) therapy for patients with a single positive sentinel lymph node (SLN) metastasis treated with a completion lymph node dissection (CLND). A second protocol in the trial evaluated the prognostic significance of using molecular markers to identify submicroscopic metastases in sentinel lymph nodes that were negative by routine pathologic analysis. The role of CLND with or without adjuvant HDI was evaluated in this group of patients. The results of the study demonstrated that adjuvant HDI offered no survival benefit for patients with a single positive SLN in terms of disease-free or overall survival. Molecular staging using polymerase chain reaction (PCR) for melanoma markers did not identify a high-risk group of patients at increased risk of melanoma recurrence. Additional treatment of these patients who were PCR-positive with either CLND alone or CLND plus HDI did not improve their survival. Additional studies from the Sunbelt Melanoma Trial helped to validate the operational standards of the SLN biopsy procedure and defined the complication rates for both SLN biopsy and CLND. A prognostic risk calculator has been developed from trial data, and the importance of different micrometastatic tumor burden measurements was reported. Although the Sunbelt Melanoma Trial did not demonstrate an improvement in survival with HDI, it is an important trial that highlights the significance of surgeon-initiated randomized clinical trials that incorporate surgical techniques, molecular biomarkers, and adjuvant therapy.




Similar content being viewed by others
References
Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early-stage melanoma. Arch Surg. 1992;127:392–9.
Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17:976–83.
Van der Velde-Zimmermann D, Roijers JF, Bouwens-Rombouts A, et al. Molecular test for the detection of tumor cells in blood and sentinel nodes of melanoma patients. Am J Pathol. 1996;149:759–64.
Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17.
Balch CM, Soong SJ, Bartolucci AA, et al. Efficacy of an elective regional lymph node dissection of 1- to 4-mm-thick melanomas for patients 60 years of age and younger. Ann Surg. 1996;224:255–63; discussion 263–6.
Scoggins CR, Ross MI, Reintgen DS, et al. Prospective multi-institutional study of reverse transcriptase polymerase chain reaction for molecular staging of melanoma. J Clin Oncol. 2006;24:2849–57.
McMasters KM, Egger ME, Edwards MJ, et al. Final results of the Sunbelt Melanoma Trial: a multi-institutional prospective randomized phase III study evaluating the role of adjuvant high-dose interferon alfa-2b and completion lymph node dissection for patients staged by sentinel lymph node biopsy. J Clin Oncol. 2016;34:1079–86.
McMasters KM, Edwards MJ, Ross MI, et al. Ulceration as a predictive marker for response to adjuvant interferon therapy in melanoma. Ann Surg. 2010;252:460–5; discussion 465–6.
Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–58.
Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol.2001;19:2370–80.
Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102:493–501.
Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–55.
Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–801.
Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–35.
Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–23.
Takeuchi H, Morton DL, Kuo C, et al. Prognostic significance of molecular upstaging of paraffin-embedded sentinel lymph nodes in melanoma patients. J Clin Oncol. 2004;22:2671–80.
Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376:2211–22.
Akbani R, Akdemir Kadir C, Aksoy BA, et al. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681–96.
Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99.
Sade-Feldman M, Yizhak K, Bjorgaard SL, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. 2018;175:998–1013 e1020.
McMasters KM, Reintgen DS, Ross MI, et al. Sentinel lymph node biopsy for melanoma: how many radioactive nodes should be removed? Ann Surg Oncol. 2001;8:192–7.
McMasters KM, Wong SL, Edwards MJ, et al. Factors that predict the presence of sentinel lymph node metastasis in patients with melanoma. Surgery. 2001;130:151–6.
Wrightson WR, Wong SL, Edwards MJ, et al. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol. 2003;10:676–80.
Chao C, Wong SL, Ross MI, et al. Patterns of early recurrence after sentinel lymph node biopsy for melanoma. Am J Surg. 2002;184:520–4; discussion 525.
McMasters KM, Chao C, Wong SL, et al. Interval sentinel lymph nodes in melanoma. Arch Surg. 2002;137:543–7; discussion 547–9.
Brown RE, Ross MI, Edwards MJ, et al. The prognostic significance of nonsentinel lymph node metastasis in melanoma. Ann Surg Oncol. 2010;17:3330–5.
Scoggins CR, Ross MI, Reintgen DS, et al. Gender-related differences in outcome for melanoma patients. Ann Surg. 2006;243:693–8; discussion 698–700.
Callender GG, Gershenwald JE, Egger ME, et al. A novel and accurate computer model of melanoma prognosis for patients staged by sentinel lymph node biopsy: comparison with the American Joint Committee on Cancer model. J Am Coll Surg. 2012;214:608–17; discussion 617–9.
Egger ME, Bower MR, Czyszczon IA, et al. Comparison of sentinel lymph node micrometastatic tumor burden measurements in melanoma. J Am Coll Surg. 2014;218:519–28.
Hao H, Xiao D, Pan J, et al. Sentinel lymph node genes to predict prognosis in node-positive melanoma patients. Ann Surg Oncol. 2017;24:108–16.
Egger ME, Xiao D, Hao H, et al. Unique genes in tumor-positive sentinel lymph nodes associated with nonsentinel lymph node metastases in melanoma. Ann Surg Oncol. 2018;25:1296–303.
Mays MP, Martin RC, Burton A, et al. Should all patients with melanoma between 1- and 2-mm Breslow thickness undergo sentinel lymph node biopsy? Cancer. 2010;116:1535–44.
Scoggins CR, Bowen AL, Martin RC II, et al. Prognostic information from sentinel lymph node biopsy in patients with thick melanoma. Arch Surg. 2010;145:622–7.
Egger ME, Callender GG, McMasters KM, et al. Diversity of stage III melanoma in the era of sentinel lymph node biopsy. Ann Surg Oncol. 2013;20:956–63.
Egger ME, Kimbrough CW, Stromberg AJ, et al. Melanoma patient-reported quality-of-life outcomes following sentinel lymph node biopsy, completion lymphadenectomy, and adjuvant interferon: results from the Sunbelt Melanoma Trial. Ann Surg Oncol. 2016;23:1019–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Kelly M McMasters reports that he serves on the Scientific Advisory Board for Elucida Oncology. He also holds patents related to melanoma gene signatures and biomarkers. Dr. Egger and Dr. Scoggins report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Egger, M.E., Scoggins, C.R. & McMasters, K.M. The Sunbelt Melanoma Trial. Ann Surg Oncol 27, 28–34 (2020). https://doi.org/10.1245/s10434-019-07828-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07828-4