Abstract
Chromogranin A, chromogranin B, and secretogranin II are acidic proteins which are stored in large dense core vesicles of neurons. An antiserum, raised against a synthetic peptide (PE-11), present in the chromogranin B molecule, and an antiserum raised against secretoneurin contained in the secretogranin II sequence, was used to localize these peptides together with chromogranin A in the human hippocampal formation. The distribution of these peptides was investigated in Alzheimer’s disease and compared to control subjects.
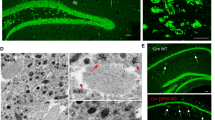
Chromogranin A, chromogranin B, and secretogranin II are distinctly distributed with an overlap in their distribution patterns. They were only detected in neuronal structures. The highest density of immunoreactivity was found for chromogranin B. A layer specific distribution was especially obvious in the inner molecular layer of the dentate gyrus as secretoneurin-like immunoreactivity was restricted to its innermost part whereas that of chromogranin B was highly concentrated throughout the inner molecular layer.
In Alzheimer’s disease, about 10 to 20% of the amyloid-immunoreactive plaques contained either chromogranin A, chromogranin B or secretoneurin. The density of secretoneurin—and chromogranin B-like immunoreactivity was significantly reduced in the inner molecular layer of the dentate gyrus, the CA1 area, the subiculum and in layers II, III and V of the entorhinal cortex.
The present study demonstrates that chromogranin peptides are markers for human hippocampal pathways. They are particularly suitable to study nerve fibers, terminating at the inner molecular layer of the dentate gyrus. Chromogranin peptides have a potential as neuronal markers for synaptic degeneration in Alzheimer’s disease.
Similar content being viewed by others
References
Arendt T. (2001) Alzheimer’s disease as a disorder of mechanisms underlying structural brain self-organization. Neuroscience 102, 723–765.
Braak H. and Braak E. (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl.) 82, 239–259.
Chanat E., Weiss U., Huttner W. B., and Tooze S. A. (1993) Reduction of the disulfide bond of chromogranin B (secretogranin I) in the trans-Golgi network causes its missorting to the constitutive secretory pathways. EMBO J 12, 2159–2168.
Davidsson P. and Blennow K. (1998) Neurochemical dissection of synaptic pathology in Alzheimer’s disease. Int. Psychogeriatr. 10, 11–23.
Folstein M. F., Folstein S. E., and McHugh P. R. (1975) “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198.
Forss-Petter S., Danielson P., Battenberg E., Bloom F., and Sutcliffe J. G. (1989) Nucleotide sequence and cellular distribution of rat chromogranin B (secretogranin I) mRNA. J. Mol. Neurosci. 1, 63–75.
Frotscher M., Seress L., Schwerdtfeger W. K., and Buhl E. (1991) The mossy cells of the fascia dentata: a comparative study of their fine structure and synaptic connections in rodents and primates. J. Comp. Neurol. 312, 145–163.
Gerdes H. H., Phillips E., and Huttner W. B. (1988) The primary structure of rat secretogranin II deduced from a cDNA sequence. Nucl. Acids Res. 16, 11,811.
Gozes I. and Brenneman D. E. (2000) A new concept in the pharmacology of neuroprotection. J. Mol. Neurosci. 14, 61–68.
Heinonen O., Soininen H., Sorvari H., Kosunen O., Paljarvi L., Koivisto E., and Riekkinen-P. J. S. (1995) Loss of synaptophysin-like immunoreactivity in the hippocampal formation is an early phenomenon in Alzheimer’s disease. Neuroscience 64, 375–384.
Huttner W. B., Gerdes H. H., and Rosa P. (1991) The granin (chromogranin/secretogranin) family. TIBS 16, 27–30.
Hyman B. T. (1998) New neuropathological criteria for Alzheimer disease. Arch. Neurol. 55, 1174–1176.
Jellinger K. A. (1997) Neuropathological staging of Alzheimer-related lesions: the challenge of establishing relations to age. Neurobiol. Aging 18, 369–375.
Jellinger K. A. and Bancher C. (1998) Neuropathology of Alzheimer’s disease: a critical update. J. Neural Transm. Suppl. 54, 77–95.
Kaufmann W. A., Barnas U., Humpel C., et al. (1998) Synaptic loss reflected by secretoneurin-like immunoreactivity in the human hippocampus in Alzheimer’s disease. Europ. J. Neurosci. 10, 1084–1094.
Kirchmair R., Hogue-Angeletti R., Gutierrez J., Fischer-Colbrie R., and Winkler H. (1993) Secretoneurin—a neuropeptide generated in brain, adrenal medulla and other endocrine tissues by proteolytic processing of secretogranin II (chromogranin C). Neuroscience 53, 359–365.
Knowles R. B., Gomez-Isla T., and Hyman B. T. (1998) Abeta associated neuropil changes: correlation with neuronal loss and dementia. J. Neuropathol. Exp. Neurol. 57, 1122–1130.
Kroesen S., Marksteiner J., Leitner B., Hogue Angeletti R., Fischer Colbrie R., and Winkler H. (1996) Rat brain: distribution of immunoreactivity of PE-11, a peptide derived from chromogranin B. Eur. J. Neurosci. 8, 2679–2689.
Lassmann H., Weiler R., Fischer P., et al. (1992) Synaptic pathology in Alzheimer’s disease: immunological data for markers of synaptic and large dense-core vesicles. Neuroscience 46, 1–8.
Mahata S. K., Mahata M., Marksteiner J., Sperk G., Fischer-Colbrie R., and Winkler H. (1991) Distribution of mRNAs for chromogranin A and B and secretogranin II in rat brain. Europ. J. Neurosci. 3, 895–904.
Mahata S. K., Mahata M., Parmer R. J., and O’Connor D. T. (1999) Desensitization of catecholamine release. The novel catecholamine release-inhibitory peptide catestatin (chromogranin A 344–364) acts at the receptor to prevent nicotinic cholinergic tolerance. J. Biol. Chem. 274, 2920–2928.
Marksteiner J., Kirchmair R., Mahata S. K., et al. (1993a) Distribution of secretoneurin, a peptide derived from secretogranin II, in rat brain: an immunocytochemical and radioimmunological study. Neuroscience 54, 923–944.
Marksteiner J., Saria A., Kirchmair R., et al. (1993b) Distribution of secretoneurin-like immunoreactivity in comparison with substance P- and enkephalin-like immunoreactivities in various human forebrain regions. Eur. J. Neurosci. 5, 1573–1585.
Marksteiner J., Bauer R., Kaufmann W. A., Weiss E., and Barnas U. M. J. (1999) PE-11, a peptide derived from chromogranin B, in the human brain. Neurosci. 91, 1155–1170.
Marksteiner J., Lechner T., Kaufmann W. A., et al. (2000) Distribution of chromogranin B-like immunoreactivity in the human hippocampus and its changes in Alzheimer’s disease. Acta Neuropathol. (Berl.) 100, 205–212.
Masliah E., Terry R. D., Alford M., DeTeresa R., and Hansen L. A. (1991) Cortical and subcortical patterns of synaptophysinlike immunoreactivity in Alzheimer’s disease. Am. J. Pathol. 138, 235–246.
McKhann G., Drachman D., Folstein M., Katzman R., Price D., and Stadlan E. M. (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944.
Munoz D. G. (1990) The distribution of chromogranin A-like immunoreactivity in the human hippocampus coincides with the pattern of resistance to epilepsy-induced neuronal damage. Ann. Neurol. 27, 266–275.
Reisberg B., Ferris S. H., De Leon M. J., and Crook T. (1982) The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 139, 1136–1139.
Salahuddin M. J., Sekiya K., Ghatei M. A., and Bloom S. R. (1989) Regional distribution of chromogranin B 420–493-like immunoreactivity in the pituitary gland and central nervous system of man, guinea-pig and rat. Neurosci. 30, 231–240.
Scheff S. W. and Price D. A. (1998) Synaptic density in the inner molecular layer of the hippocampal dentate gyrus in Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 57, 1146–1153.
Solodkin A. and Van Hoesen G. W. (1996) Entorhinal cortex modules of the human brain. J. Comp. Neurol. 365, 610–617.
Somogyi P., Hodgson A. J., DePotter R. W., et al. (1984) Chromogranin immunoreactivity in the central nervous system. Immunochemical characterisation, distribution and relationship to catecholamine and enkephalin pathways. Brain Res. 320, 193–230.
Thiele C. and Huttner W. B. (1998) The disulfide-bonded loop of chromogranins, which is essential for sorting to secretory granules, mediates homodimerization. J. Biol. Chem. 273, 1223–1231.
Winkler H. and Fischer-Colbrie R. (1992) The chromogranins A and B: The first 25 years and future perspectives. Neuroscience 49, 497–528.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marksteiner, J., Kaufmann, W.A., Gurka, P. et al. Synaptic proteins in Alzheimer’s disease. J Mol Neurosci 18, 53–63 (2002). https://doi.org/10.1385/JMN:18:1-2:53
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:18:1-2:53