Abstracts
Giant pituitary adenomas are uncommonly large tumors, greater than 4 cm in size that can produces endocrine symptoms, visual loss and cranial nerve palsies. We report the rare occurrence of seesaw nystagmus as the presenting sign of giant pituitary adenoma. A 50-year-old man presented with headache associated with visual loss and seesaw nystagmus. Perimetry revealed bitemporal hemianopia and magnetic resonance imaging showed a giant pituitary adenoma. After surgery, nystagmus disappeared. Our case is relevant in understanding its pathogenesis since it documents seesaw nystagmus in a patient bitemporal hemianopia due to a large tumor but without mesencephalic compression.
pituitary tumor; giant pituitary adenoma; bitemporal hemianopia; nystagmus; seesaw nystagmus
Adenoma pituitário gigante é um tumor incomum, maior que 4 cm que produz sintomas endócrinos, perda visual e paralisia de nervos cranianos. Relatamos um caso de nistagmo em gangorra como sinal de apresentação de adenoma pituitário gigante. Um paciente de 50 anos, masculino, apresentava cefaléia, perda visual e nistagmo em gangorra. A perimetria revelou hemianopsia bitemporal e a imagem por ressonância magnética demonstrou um adenoma pituitário gigante. Após a cirurgia, o nistagmo desapareceu. Nosso caso é importante na compreensão da fisiopatogenia do nistagmo em gangorra, pois documenta sua ocorrência em paciente com hemianopsia bitemporal decorrente de tumor hipofisário sem compressão mesencefálica.
tumor pituitário; adenoma pituitário gigante; hemianopsia bitemporal; nistagmo; nistagmo em gangorra
Seesaw nystagmus caused by giant pituitary adenoma: case report
Nistagmo em gangorra causado por adenoma pituitário gigante: relato de caso
Frederico Castelo MouraI; Allan Christian Pieroni GonçalvesI; Mário Luiz Ribeiro MonteiroII
Division of Ophthalmology, Hospital das Clínicas of the University of São Paulo Medical School, São Paulo SP, Brazil
IFellow
IIAssistant Professor
ABSTRACT
Giant pituitary adenomas are uncommonly large tumors, greater than 4 cm in size that can produces endocrine symptoms, visual loss and cranial nerve palsies. We report the rare occurrence of seesaw nystagmus as the presenting sign of giant pituitary adenoma. A 50-year-old man presented with headache associated with visual loss and seesaw nystagmus. Perimetry revealed bitemporal hemianopia and magnetic resonance imaging showed a giant pituitary adenoma. After surgery, nystagmus disappeared. Our case is relevant in understanding its pathogenesis since it documents seesaw nystagmus in a patient bitemporal hemianopia due to a large tumor but without mesencephalic compression.
Key words: pituitary tumor, giant pituitary adenoma, bitemporal hemianopia, nystagmus, seesaw nystagmus.
RESUMO
Adenoma pituitário gigante é um tumor incomum, maior que 4 cm que produz sintomas endócrinos, perda visual e paralisia de nervos cranianos. Relatamos um caso de nistagmo em gangorra como sinal de apresentação de adenoma pituitário gigante. Um paciente de 50 anos, masculino, apresentava cefaléia, perda visual e nistagmo em gangorra. A perimetria revelou hemianopsia bitemporal e a imagem por ressonância magnética demonstrou um adenoma pituitário gigante. Após a cirurgia, o nistagmo desapareceu. Nosso caso é importante na compreensão da fisiopatogenia do nistagmo em gangorra, pois documenta sua ocorrência em paciente com hemianopsia bitemporal decorrente de tumor hipofisário sem compressão mesencefálica.
Palavras-chave: tumor pituitário, adenoma pituitário gigante, hemianopsia bitemporal, nistagmo, nistagmo em gangorra.
Pituitary tumors are common diseases representing about 12% of intracranial tumors1. While microadenomas are located within the sella and do not cause visual loss, macroadenomas are tumors with larger diameters (>10 mm) that extend beyond the limits of the sella and may cause neuro-ophthalmologic manifestations by the compression of adjoining structures1. A less common variant is giant pituitary adenoma, first described by Jefferson2 and characterized by its large dimensions, reaching diameters of more than four centimeters2,3. Enlargement of the tumor usually occurs in an upward direction, due to the low resistance of the sellar diaphragm, assailing the intracranial portion of the optic nerve, the chiasm and the optic tract, causing a progressive visual loss. Lateral tumor growth may also occur invading the medial cranial fossa and/ or cavernous sinus and possibly causing lesion of the oculomotor, trochlear, abducent, trigeminal nerves, and the sympathetic ocular innervation2,3.
The purpose of this paper is to describe a patient with a giant pituitary adenoma that presented with seesaw nystagmus, one of very rare manifestations of this type of tumor. We also review other cases of sewsaw nystagmus and discuss its pathogenesis in patients with pituitary adenomas.
CASE
A 50-year-old man came to the Ophthalmology Service of Hospital das Clínicas of University of São Paulo Medical School, complaining of a one-year history of bilateral temporal visual loss, tremor in both eyes, and "multiplication" and vertical motion of images. He also referred holocranial headache for the past 10 years.
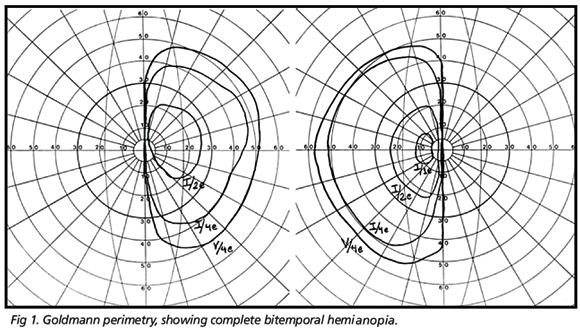
On ophthalmic examination best corrected visual acuity was 20/70 in the right eye (OD) and 20/80 in the left eye (OS). Pupils were equal in size and there was no relative afferent pupillary defect. Slit lamp examination and applanation tonometry revealed findings within normal limits. During observation of the ocular motility, the patient displayed vertical and rotary nystagmus (seesaw nystagmus), which increased in amplitude and diminished in frequency when the patient looked right or left. The oscillation was continuous, presenting both vertical and torsional component. Nystagmus was of medium frequency, without a quick phase and displaying continuous alternation between eyes; when one went up, the other came down and vice-versa. Ophthalmoscopy revealed optic disc pallor and retinal nerve fiber layer loss in both eyes and persistence of myelinated fibers in the OS. Goldmann perimetry demonstrated bitemporal hemianopsy, with complete loss of the temporal field of each eye (Fig 1). Magnetic resonance imaging (MRI) revealed a giant pituitary tumor with sellar and supra-sellar extension and compression of the supra-sellar cistern and optic chiasm. Neither the cerebral parenchyma nor the mesencephalon were compressed by the tumor (Fig 2).
The patient was submitted to trans-esphenoidal tumor removal that was uneventful. There was considerable visual improvement with complete recovery of the visual acuity and marked improvement of visual fields. A few months later, there was complete disappearance of seesaw nystagmus.
DISCUSSION
Seesaw nystagmus is a type of pendular nystagmus where a half cycle consists of the elevation and intorsion of one eye, concurrently with the depression and extortion of the fellow eye. In the other half cycle, there is an inversion of the ocular movements4. The first description of seesaw nystagmus was made by Maddox5 in a patient with bitemporal hemianopia of unknown origin. In reviewing the literature, we noted that many of the cases of seesaw nystagmus were associated with extrinsic suprasellar lesions (e.g., craniopharyngioma and pituitary adenoma) compressing or invading the mesodiencephalic region. The majority of such cases displayed visual symptoms including bitemporal hemianopia. Vascular illnesses of the brainstem, such as infarction, also are known causes of this type of nystagmus, but without the bitemporal hemianopsy. In a few cases, the focal lesion of the central nervous system was not identified. Included in this group are patients in whom seesaw nystagmus was congenital6, associated with ocular abnormalities7 and post-traumatic8. The association of seesaw nystagmus with giant pituitary adenoma in the past was reported by very few authors9-14. All patients in these accounts displayed poor visual acuity and bitemporal hemianopsy.
The pathogenesis of seesaw nystagmus remains unknown; however, based on previous accounts, some assumptions have been put forth. Due to its frequent association with visual symptoms, especially bitemporal hemianopsy it was suggested that visual deprivation might be the cause of seesaw nystagmus15-19. Nakada and Kwee20 added that retinal error signals reach the inferior olivary nucleus, in the chiasmal region, caused unbalance in the vestibulo-ocular reflex adaptation. Another condition that seems to be important in the genesis of seesaw nystagmus is preservation of geniculocortical function. This theory is based on the fact that, in a state of absolute blindness, seesaw nystagmus does not occur and disappears when the eyes are closed and/or are in the dark20.
On the other hand, due to seesaw nystagmus having been reported in patients with lesions of the brainstem, in the absence of any visual deficit, some authors21,22 believe that its pathogenesis may be due to dysfunction of the ocular counterrolling system, and not to disturbance in visual input. Studies have established that jerk seesaw nystagmus (with the torsional component of the quick phases beating toward the side of the mesodiencephlic lesion) can occur in patients with lesions in the region of intersticial nucleus of Cajal (INC), adjoining the mesodiencephalic joint21,23-25. It is believed that jerky seesaw nystagmus may occur due to imbalance of central otolithic projections from vestibular nuclei to the INC. There are papers in which it is shown that lesions in the region of INC are able to cause a contralateral ocular tilt reaction26,27. The relevance of these observations to seesaw nystagmus mechanism is that the latters half cycle is identical to ocular motion in the ocular tilt reaction.
Since mesodiencephalic lesions are capable of causing seesaw nystagmus, many authors suggest that, in giant adenomas accompanied by this type of nystagmus, there would be, in addition to loss of vision, compression also of the midbrain by the tumor24. The current case is important in that it has been studied by MRI, a neurological imaging method not to be found documented in any other similar case. This study made it clear that the tumor did not compress the midbrain, despite its large suprasellar growth (Fig 2). Following excision of the tumor, there was improved acuity and visual field and complete resolution of the nystagmus. This case serves, therefore, to indicate that, in giant pituitary adenomas, disturbance of visual input has been thought to play a main role in the seesaw nystagmus pathogenesis.
In concluding, this paper emphasizes the importance of seesaw nystagmus being recognized as one of the most uncommon manifestations of pituitary adenomas. This study helps in understanding the pathogenesis of such kind of nystagmus, documenting by MRI, that the tumor did compress the optical chiasm, but did not compress the mesencephalon region, indicating that that loss of the visual field alone may be the foremost factor in producing seesaw nystagmus in large pituitary adenomas.
4. Miller NR, Newman NJ. Nystagmus and related ocular motility disorders. In Walsh & Hoyts clinical neuro-ophthalmology, 5th ed. Baltimore: Willians & Wilkins, 1998:1477-1478.
Received 17 June 2005. Accepted 4 October 2005.
Dr. Frederico Castelo Moura - Rua Oscar Freire 1799 / 1307 - 05409-011 São Paulo SP - Brasil. E-mail: fredcastelo@terra.com.br
- 1. Anderson D, Faber P, Marcovitz S, et al. Pituitary tumors and the ophthalmologist. Ophthalmology 1983;90:1265-1270.
- 2. Jefferson G. Extrasellar extension of pituitary adenoma. Proc R Soc Med 1940;33:433-458.
- 3. Grote E. Characteristics of giant pituitary adenomas. Acta Neurochir (Wien) 1982;60:141-153.
- 5. Maddox E. Seesaw nystagmus with bitemporal hemianopsia. Proc R Soc Med 1914;7:12-13.
- 6. Schmidt D, Kommerell G. [Congenital seewaw nystagmus (author's transl)]. Albrecht Von Graefes Arch Klin Exp Ophthalmol 1974;191: 265-272.
- 7. Rucker C. Seesaw nystagmus associated with choroiditis and positive neutralization test for toxoplasmosis. Arch Ophthalmology 1946;35: 301-302.
- 8. Frisen L, Wikkelso C. Posttraumatic seesaw nystagmus abolished by ethanol ingestion. Neurology 1986;36:841-844.
- 9. Lourie H. Seesaw nystagmus: case report elucidating the mechanism. Arch Neurol 1963;9:531-533.
- 10. Schurr PH. Seesaw nystagmus. Proc R Soc Med 1963;56:808-810.
- 11. Daroff RB. Seesaw Nystagmus. Neurology 1965;15:874-877.
- 12. Druckman R, Ellis P, Kleinfeld J, Waldman M. Seesaw nystagmus. Arch Ophthalmol 1966;76:668-675.
- 13. Safran AB, Berney J, Panchaud A. [Seesaw nystagmus in a case of giant adenoma of the hypophysis]. Rev Med Suisse Romande 1980;100: 343-347.
- 14. Rossazza C, Delplace MP, Larmande A. [The seesaw nystagmus in hypophyseal adenomas]. Rev Otoneuroophtalmol 1974;46:367-368.
- 15. Leitch RJ, Thompson D, Harris CM, et al. Achiasmia in a case of midline craniofacial cleft with seesaw nystagmus. Br J Ophthalmol 1996; 80:1023-1024.
- 16. May EF, Truxal AR. Loss of vision alone may result in seesaw nystagmus. J Neuroophthalmol 1997;17:84-85.
- 17. Davis GV, Shock JP. Septo-optic dysplasia associated with seesaw nystagmus. Arch Ophthalmol 1975;93:137-139.
- 18. Arnott EJ. Vertical seesaw nystagmus. Trans Ophthalmol Soc U K 1964;84:251-257.
- 19. Slatt B, Nykiel F. Seesaw Nystagmus. Am J Ophthalmol 1964;58: 1016-1021.
- 20. Nakada T, Kwee IL. Seesaw nystagmus: role of visuovestibular interaction in its pathogenesis. J Clin Neuroophthalmol 1988;8:171-177.
- 21. Halmagyi GM, Aw ST, Dehaene I, et al. Jerk-waveform seesaw nystagmus due to unilateral meso-diencephalic lesion. Brain 1994;117 (Pt4): 789-803.
- 22. Williams IM, Dickinson P, Ramsay RJ, Thomas L. Seesaw nystagmus. Aust J Ophthalmol 1982;10:19-25.
- 23. Brandt T, Dieterich M. Vestibular syndromes in the roll plane: topographic diagnosis from brainstem to cortex. Ann Neurol 1994;36:337-347.
- 24. Halmagyi GM, Hoyt WF. Seesaw nystagmus due to unilateral mesodiencephalic lesion. J Clin Neuroophthalmol 1991;11:79-84.
- 25. Halmagyi GM, Brandt T, Dieterich M, et al. Tonic contraversive ocular tilt reaction due to unilateral meso-diencephalic lesion. Neurology 1990;40:1503-1509.
- 26. Westheimer G, Blair SM. The ocular tilt reaction--a brainstem oculomotor routine. Invest Ophthalmol 1975;14:833-839.
- 27. Lueck CJ, Hamlyn P, Crawford TJ, et al. A case of ocular tilt reaction and torsional nystagmus due to direct stimulation of the midbrain in man. Brain 1991;114(Pt5):2069-2079.
Publication Dates
-
Publication in this collection
06 Apr 2006 -
Date of issue
Mar 2006
History
-
Accepted
04 Oct 2005 -
Received
17 June 2005