
Sodium-glucose cotransporter-2 inhibitors protect against atrial fibrillation in patients with heart failure
Introduction
Cardiac structural or functional damage can result in heart failure (HF) which is characterized by a series of typical symptoms and signs including dyspnea, fatigue, ankle swelling, and limited physical capacity, with preserved left ventricular ejection fraction and reduced ejection fraction (1,2). Globally, millions of patients suffer from HF and it is associated with poor quality of life, high morbidity and mortality, as well as substantial medical costs to society (3). Atrial fibrillation (AF) is the most common arrhythmia in HF patients and occurs in more than half of all individuals with HF (2).
AF is associated with several pathophysiological mechanisms, including atrial electrical, structural remodeling, and glycemic fluctuations (3). In such patients, AF increases the risk of hospitalization, stroke, and all-cause mortality (4). AF and HF commonly coexist and the prevalence of both conditions is expected to rise with the aging population world-wide (5). Despite a plethora of advances in the field of AF and HF over the past 2 decades (6), it remains uncertain which medications can provide optimal long-term outcome for patients with coexisting AF and HF.
The sodium-glucose cotransporter-2 (SGLT-2) is mainly expressed in the kidneys and is responsible for the reabsorption of sodium (Na+) and glucose in the renal tubules (7). Inhibition of SGLT-2 increases the urinary glucose excretion and augments natriuresis in patients with type 2 diabetes (T2DM) (8). Thus, SGLT-2 inhibitors have emerged as novel glucose-lowering medications. Furthermore, SGLT-2 inhibitors have been shown to be beneficial for HF hospitalizations in T2DM patients by lowering cardiac pre-load and reducing pulmonary congestion (9,10). In fact, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) both recommend the use of SGLT-2 inhibitors in T2DM patients with HF (11). In addition, two large international randomized trials demonstrated that SGLT-2 inhibitors can significantly lower the risk of deteriorating HF, as well as reducing cardiovascular mortality in HF patients with reduced ejection fraction (HFrEF) (12,13). Recently, evidence of a real-world study showed that SGLT-2 inhibitors reduced the risk of new-onset AF in T2DM patients, and subgroup analysis revealed that the use of SGLT-2 inhibitors was associated with a lower risk of new-onset AF in T2DM patients with congestive heart failure compared to those without congestive heart failure (14). Furthermore, studies have shown that SGLT-2 inhibitors can also promote weight loss, lower blood pressure, and reduce inflammation and oxidative stress independent of the effect on blood glucose (7). Since oxidative stress and inflammation play essential roles in the development and progression of AF (15), SGLT-2 inhibitors may be associated with the risk of AF in HF patients. Therefore, this study investigated the association between SGLT-2 inhibitors and the risk of AF in patients with HF. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-2694).
Methods
Study participants
In this retrospective observational study, patients aged 18 years and over, who were admitted to the First Affiliated Hospital of Anhui Medical University for HF between January 2016 and December 2020, were recruited. The diagnose of HF was determined by systematic medical history review, physical examination, and laboratory tests. The diagnose of AF was determined by medical history and electrocardiogram which was performed after the initial consultation. Patients with unknown cardiomyopathy, uncontrolled arrhythmia, mental disorders, malignant carcinoma, or severe kidney or liver dysfunction were excluded from the study. Finally, a total of 903 patients with HF were enrolled, including 78 participants with AF and 825 participants without AF. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of the First Affiliated Hospital of Anhui Medical University (No. 2016-004) and informed consent was taken from all the patients.
Data collection
Basic patient data including age, gender, weight, height, smoking status, systolic blood pressure (SBP), diastolic blood pressure (DBP), previous medical history, and cardiovascular medications were collated via a standardized data collection form. The main SGLT-2 inhibitors used were dapagliflozin, empagliflozin, and canagliflozin. HF was classified according to the New York Heart Association (NYHA) classification system (16), however, the type of AF was not classified.
Venous blood samples were collected from patients after a 10-hour overnight fast. Samples were tested for levels of hemoglobin A1c (HbA1c), triglyceride (TG), low density lipoprotein cholesterol (LDL-C), uric acid (UA), estimated glomerular filtration rate (eGFR), and B-type natriuretic peptide (BNP). All tests were performed in our hospital.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD), or median (interquartile range, IQR). Continuous variables were compared using Student’s t-tests or nonparametric tests. Categorical variables are presented as frequencies and percentages and compared using the Chi-square test among different groups. The study participants with HF were divided into two groups based on the AF status and the NYHA classification. Logistic regression analysis was used to evaluate the independent association of SGLT-2 inhibitors and the risk of AF in HF patients, after controlling for potential confounders including age, gender, BMI, duration of HF, coronary heart disease, cerebrovascular disease, diabetes, cardiovascular medication, blood pressure, HbA1c, UA, eGFR, and BNP. The effect of SGLT-2 inhibitors on AF was also assessed according to subgroups based on age, gender, BMI, eGFR, and NYHA classification. Two-tailed P values <0.05 were considered statistically significant. Statistical analysis was performed using the SPSS Statistics 21.0 software (IBM SPSS, Armonk, NY).
Results
Basic characteristics of participants by AF status and NYHA classification
A total of 903 participants, including 412 (45.6%) females, were enrolled in this study. The mean age of the patients was 64.6±9.5 years and the mean BMI was 24.1±4.7 kg/m2. Table 1 shows the clinical characteristics of patients with AF compared to patients without AF. Patients with AF were older and predominantly male and had a higher prevalence of coronary heart disease, cerebrovascular disease, and diabetes. In addition, AF patients used beta-blockers, anticoagulants, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin II receptor blockers (ARB) more frequently than individuals without AF. Patients with AF were more likely to have a higher BMI, blood pressure, UA, eGFR, and BNP compared to patients without AF. There were no differences in the smoking history, use of antiplatelet agents, nor levels of HbA1c, TG, and LDL-C between the two groups.
Table 1
| Indexes | AF group (N=78) | No AF group (N=825) | P value |
|---|---|---|---|
| Age, years | 65.6±6.7 | 63.4±6.4 | <0.001 |
| Female, n (%) | 34 (43.6) | 378 (45.8) | <0.001 |
| Smoking, n (%) | 17 (21.8) | 126 (15.3) | 0.117 |
| BMI, kg/m2 | 25.4±4.2 | 23.7±3.6 | 0.002 |
| Duration of HF, years | 6.5±2.1 | 4.3±3.7 | 0.009 |
| History of coronary heart disease, n (%) | 35 (44.9) | 218 (26.4) | <0.001 |
| History of cerebrovascular disease, n (%) | 11 (14.1) | 58 (7.0) | 0.014 |
| History of diabetes, n (%) | 18 (23.1) | 115 (14.0) | 0.021 |
| Cardiovascular medication, n (%) | |||
| Beta-blocker | 62 (79.5) | 486 (58.9) | <0.001 |
| ACE inhibitors or ARB | 65 (83.3) | 618 (74.9) | 0.031 |
| Antiplatelet | 35 (44.9) | 388 (47.0) | 0.314 |
| Anticoagulant | 51 (65.4) | 16 (1.9) | <0.001 |
| SBP, mmHg | 129±17 | 135±11 | <0.001 |
| DBP, mmHg | 69±10 | 74±13 | 0.012 |
| HbA1c, % | 7.6±2.0 | 7.4±1.7 | 0.087 |
| TG, mmol/L | 1.69 (0.79, 2.04) | 1.77 (0.91, 2.21) | 0.176 |
| LDL-C, mmol/L | 2.71±1.05 | 2.47±1.22 | 0.214 |
| UA, μmol/L | 371±89 | 322±91 | <0.001 |
| eGFR, mL/min/1.73 m2 | 79.8±14.2 | 88.2±15.8 | 0.003 |
| BNP, pg/mL | 1,801±304 | 1,208±279 | <0.001 |
AF, atrial fibrillation; BMI, body mass index; HF, heart failure; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blockers; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; UA, uric acid; eGFR, estimated glomerular filtration rate; BNP, B-type natriuretic peptide.
Patients were categorized according to the NYHA classification, namely, the NYHA I/II group and the NYHA III/IV group. Table 2 summarizes the characteristics of study participants by NYHA classification. In general, HF patients with NYHA III/IV were older and heavier. There were also more likely to have a longer duration of HF, a history of coronary heart disease, a higher level of blood pressure, and higher levels of LDL-C and BNP compared to subjects with NYHA III/IV. In contrast, HF patients with NYHA III/IV had lower levels of eGFR. However, patients in the NYHA I/II group and the NYHA III/IV group had comparable circulating concentrations of HbA1c, TG, and UA.
Table 2
| Indexes | NYHA I/II (N=723) | NYHA III/IV (N=180) | P value |
|---|---|---|---|
| Age, years | 66.1±6.2 | 68.4±7.7 | <0.001 |
| Female, n (%) | 332 (45.9) | 80 (44.4) | 0.301 |
| Smoking, n (%) | 122 (16.9) | 21 (11.7) | 0.116 |
| BMI, kg/m2 | 23.7±3.2 | 24.5±3.9 | 0.021 |
| Duration of HF, years | 4.7±2.9 | 6.4±3.1 | <0.001 |
| History of coronary heart disease, n (%) | 196 (27.1) | 57 (31.7) | <0.001 |
| History of cerebrovascular disease, n (%) | 60 (8.3) | 9 (5.0) | 0.247 |
| History of diabetes, n (%) | 98 (13.6) | 35 (19.4) | 0.081 |
| Cardiovascular medication, n (%) | |||
| Beta-blocker | 442 (61.1) | 106 (58.9) | 0.412 |
| ACE inhibitors or ARB | 558 (77.2) | 125 (69.4) | 0.034 |
| Antiplatelet | 306 (40.9) | 117 (65.0) | <0.001 |
| Anticoagulant | 34 (4.7) | 33 (18.3) | <0.001 |
| SBP, mmHg | 129±15 | 134±17 | 0.009 |
| DBP, mmHg | 70±11 | 75±12 | 0.027 |
| HbA1c, % | 7.4±1.3 | 7.7±1.8 | 0.081 |
| TG, mmol/L | 1.77 (0.82–2.09) | 1.74 (0.94–2.17) | 0.325 |
| LDL-C, mmol/L | 2.55±1.41 | 2.17±1.20 | 0.011 |
| UA, μmol/L | 342±77 | 355±81 | 0.112 |
| eGFR, mL/min/1.73 m2 | 86.5±11.7 | 79.6±13.7 | 0.007 |
| BNP, pg/mL | 1308±401 | 1798±311 | <0.001 |
NYHA, New York Heart Association; AF, atrial fibrillation; BMI, body mass index; HF, heart failure; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blockers; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; UA, uric acid; eGFR, estimated glomerular filtration rate; BNP, B-type natriuretic peptide.
Risk for AF with SGLT-2 inhibitors
The prevalence of AF increased with ascending NYHA classification (Figure 1A; P=0.016). The incidence of AF in patients with NYHA I classification was 8.1%, compared to 10.2% in NYHA II patients, 14.2% in NYHA III patients, and 15.8% in the NYHA IV group. Administration of SGLT-2 inhibitors significantly lowered the prevalence of AF compared to patients who did not use SGLT-2 inhibitors (8.4% vs. 12.1%, P<0.001) (Figure 1B). After controlling for potential confounders, logistic regression analysis revealed that the use of SGLT-2 inhibitors was independently associated with a lower risk of AF (Table 3). Model 1 shows the analysis adjusted for age and gender, and Model 2 shows the results adjusted for age, gender, BMI, duration of HF, coronary heart disease, cerebrovascular disease, diabetes, and cardiovascular medication. Model 3 represents model 2 further adjusted for blood pressure, HbA1c, UA, eGFR, and BNP (OR =0.76; 95% CI: 0.70–0.85; P<0.001).
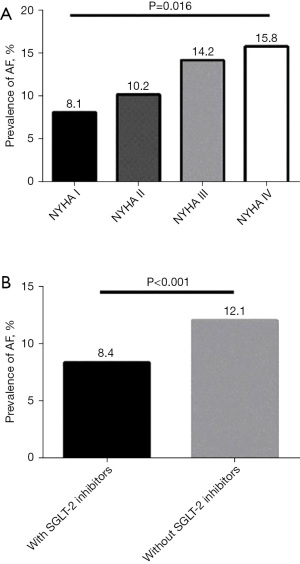
Table 3
| Without SGLT-2 inhibitors | With SGLT-2 inhibitors | P value | ||
|---|---|---|---|---|
| ORs | 95% CI | |||
| Univariate analysis | 1.00 (Reference) | 0.64 | 0.60–0.72 | <0.001 |
| Model 1 | 1.00 (Reference) | 0.68 | 0.64–0.75 | <0.001 |
| Model 2 | 1.00 (Reference) | 0.72 | 0.67–0.77 | <0.001 |
| Model 3 | 1.00 (Reference) | 0.76 | 0.70–0.85 | <0.001 |
Model 1 is adjusted for age and gender. Model 2 is adjusted for age, gender, BMI, duration of HF, coronary heart disease, cerebrovascular disease, diabetes, and cardiovascular medication. Model 3 is adjusted for all the factors in Model 2, plus blood pressure, HbA1c, UA, eGFR, and BNP. BMI, body mass index; HF, heart failure; HbA1c, hemoglobin A1c; UA, uric acid; eGFR, estimated glomerular filtration rate; BNP, B-type natriuretic peptide; SGLT-2, sodium-glucose co-transporter 2.
Subgroup analysis
The effect of SGLT-2 inhibitors on AF was further analyzed according to subgroups (Table 4). The effect of SGLT-2 inhibitors on AF in patients aged <65 years was comparable to that of patients aged ≥65 years (OR =0.82 and 95% CI: 0.71–0.88 vs. OR =0.84 and 95% CI: 0.77–0.92; P=0.501). Similarly, neither gender, BMI, nor the level of eGFR had any consequence on the effect of SGLT-2 inhibitors on AF. Moreover, the effect of SGLT-2 inhibitors on AF was not modified by NYHA classification (OR =0.76 and 95% CI: 0.70–0.85 for NYHA I/II vs. OR =0.73 and 95% CI: 0.67–0.89 for NYHA III/IV; P interaction =0.104).
Table 4
| Subgroups | Without SGLT-2 inhibitors | With SGLT-2 inhibitors | P Interaction | |
|---|---|---|---|---|
| ORs | 95% CI | |||
| Age | 0.501 | |||
| <65 years | 1.00 (Reference) | 0.82 | 0.71–0.88 | |
| ≥65 years | 1.00 (Reference) | 0.84 | 0.77–0.92 | |
| Gender | 0.217 | |||
| Male | 1.00 (Reference) | 0.79 | 0.70–0.87 | |
| Female | 1.00 (Reference) | 0.83 | 0.76–0.90 | |
| BMI, kg/m2 | 0.341 | |||
| <28 | 1.00 (Reference) | 0.75 | 0.70–0.83 | |
| ≥28 | 1.00 (Reference) | 0.80 | 0.76–0.88 | |
| eGFR, mL/min/1.73 m2 | 0.612 | |||
| ≥90 | 1.00 (Reference) | 0.80 | 0.72–0.89 | |
| 60–90 | 1.00 (Reference) | 0.77 | 0.70–0.87 | |
| <60 | 1.00 (Reference) | 0.74 | 0.67–0.82 | |
| NYHA classification | 0.104 | |||
| I/II | 1.00 (Reference) | 0.76 | 0.70–0.85 | |
| III/IV | 1.00 (Reference) | 0.73 | 0.67–0.89 | |
SGLT-2, sodium-glucose co-transporter 2; BMI, body mass index; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; SGLT-2, sodium-glucose co-transporter 2.
Discussion
This observational study demonstrated that SGLT-2 inhibitors were independently associated with a decreased risk of AF among patients with HF. After adjusting for potential confounders, the effect of SGLT-2 inhibitors on AF remained significant. Of note, the effect of SGLT-2 inhibitors was found to be consistent in patients aged <65 years and patients aged ≥65 years. In addition, NYHA classification did not modify the effect of SGLT-2 inhibitors on AF in patients with HF. The ability of SGLT-2 inhibitors to reduce the incidence of AF was independent of gender, BMI, and eGFR.
HF has been identified as an independent risk factor for AF, possibly by inducing atrial fibrosis and atrial ionic remodeling (17). Individuals with HF and AF have increased risk of cardiovascular complications (4). Both hypertensive heart disease and diabetes mellitus are commonly observed in HF patients, and are associated with a higher prevalence of AF. The possible mechanisms causally linking HF with AF is complex, and may include mechanical stress, inflammation, and electrical remodeling (6).
SGLT-2 inhibitors suppress the active reabsorption of sodium and glucose at the level of the proximal renal tubule, and this may lead to a reduction in blood pressure, blood glucose, and body weight (18). In the cardiovascular outcome trials, dapagliflozin was shown to reduce the worsening of HF events in patients with HF (12), while empagliflozin reduced the risk of cardiovascular death or hospitalization for HF (13). However, there is currently a paucity of data suggesting a relationship between SGLT-2 inhibitors and AF in HF patients. This study demonstrated the protective effect of SGLT-2 inhibitors against AF in patients with HF.
To date, there is conflicting data regarding the protective effect of SGLT-2 inhibitors. A systematic review and meta-analysis (n=3,157,259) conducted by Li et al. concluded that SGLT-2 inhibitors did not reduce the risk of AF in patients with T2DM compared to other glucose lowering medications (19). Similarly, the CVD-REAL Nordic study, a multinational observational analysis, found no significant difference between SGLT2 inhibitors and other anti-hyperglycemic medications in terms of the incidence of AF in patients with T2DM (20). In contrast, a meta-analysis conducted by Okunrintemi et al. involving eight cardiovascular and renal outcomes, showed that SGLT-2 inhibitors significantly lowered the incidence of AF regardless of the presence or absence of diabetes (relative risk: 0.79; 95% CI: 0.67–0.93) (21). In a recent study of pharmacovigilance databases, AF was reported more frequently in patients taking diabetic drugs compared to patients taking SGLT2 inhibitors (22). In view of these conflicting results, more real-world data are required to verify the protective effect of SGLT-2 inhibitors against AF and the clinical feasibility of SGLT-2 inhibitors warrants further investigation.
Several potential mechanisms have been proposed for the protective effect of SGLT-2 inhibitors against AF in patients with HF. SGLT-2 inhibitors can protect the heart from glucotoxicity, as well as reduce the pre-load, decongestion, and filling pressures through peculiar diuretic actions, which may further reduce atrial dilation. SGLT-2 inhibitors may exert a protective effect by improving atrial structural and electrical remodeling and ameliorating mitochondrial function (23). A recent retrospective analysis demonstrated that SGLT-2 inhibitors have beneficial effects on ventricular repolarization indices including the QT interval and the T peak-to-end (Tp-e)/QT ratio (24), which may be protective against AF occurrence. Clinical data have also demonstrated that SGLT-2 inhibitors are associated with a reduction in epicardial adipose tissue volume (25), which is a biologically highly active tissue that contributes to the development and progression of AF (26). Furthermore, SGLT-2 inhibitors may affect calcium (Ca2+) cycling, Na+ balance, and inflammatory and energy balance, all of which play essential roles in the incidence and severity of AF (27).
To the best of our knowledge, this is the first study to evaluate the effect of SGLT-2 inhibitors on AF in HF patients. There were some limitations to this investigation. First, as the study did not distinguish between persistent AF and paroxysmal AF, it remains unclear whether the protective effect of SGLT-2 inhibitors might be affected by the type of AF. Second, the lack of serial electrocardiograms and Holter monitoring means it was not possible to quantify the burden of AF. Third, we regarded the BNP other than NT-proBNP as the biomarker of HF in this study. Compared with BNP, the NT-proBNP is mainly filtered by glomerulus, thus the NT-proBNP concentrations is greatly affected by renal function. HF patients may have a decrease in estimated glomerular filtration rate, so we chose the BNP other than NT-proBNP. Fourth, a causal relationship between SGLT-2 inhibitor use and AF risk could not be established due to the observational nature of this study design. Future randomized clinical trials are warranted to further investigate the protective effect of SGLT-2 inhibitors on the incidence of AF.
In conclusion, SGLT-2 inhibitors reduced the risk of AF in patients with HF, and this protective effect was not altered by age, gender, BMI, eGFR, nor NYHA classification.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-2694
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-2694
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-2694). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of the First Affiliated Hospital of Anhui Medical University (No. 2016-004) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 2005;26:1115-40. [Crossref] [PubMed]
- Lewis EF, Lamas GA, O'Meara E, et al. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail 2007;9:83-91. [Crossref] [PubMed]
- . GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. [Crossref] [PubMed]
- Santhanakrishnan R, Wang N, Larson MG, et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation 2016;133:484-92. [Crossref] [PubMed]
- Verma A, Kalman JM, Callans DJ. Treatment of Patients With Atrial Fibrillation and Heart Failure With Reduced Ejection Fraction. Circulation 2017;135:1547-63. [Crossref] [PubMed]
- Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020;141:e139-596. [Crossref] [PubMed]
- Carlisle MA, Fudim M, DeVore AD, et al. Heart Failure and Atrial Fibrillation, Like Fire and Fury. JACC Heart Fail 2019;7:447-56. [Crossref] [PubMed]
- Zelniker TA, Braunwald E. Mechanisms of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:422-34. [Crossref] [PubMed]
- Alicic RZ, Neumiller JJ, Johnson EJ, et al. Sodium-Glucose Cotransporter 2 Inhibition and Diabetic Kidney Disease. Diabetes 2019;68:248-57. [Crossref] [PubMed]
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2019;380:347-57. [Crossref] [PubMed]
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017;377:644-57. [Crossref] [PubMed]
- Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:487-93. [Crossref] [PubMed]
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019;381:1995-2008. [Crossref] [PubMed]
- Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med 2020;383:1413-24. [Crossref] [PubMed]
- Karam BS, Chavez-Moreno A, Koh W, et al. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol 2017;16:120. [Crossref] [PubMed]
- Caraballo C, Desai NR, Mulder H, et al. Clinical Implications of the New York Heart Association Classification. J Am Heart Assoc 2019;8:e014240 [Crossref] [PubMed]
- Mene-Afejuku TO, López PD, Akinlonu A, et al. Atrial Fibrillation in Patients with Heart Failure: Current State and Future Directions. Am J Cardiovasc Drugs 2018;18:347-60. [Crossref] [PubMed]
- Lăcătușu CM, Grigorescu ED, Stătescu C, et al. Association of Antihyperglycemic Therapy with Risk of Atrial Fibrillation and Stroke in Diabetic Patients. Medicina (Kaunas) 2019;55:592. [Crossref] [PubMed]
- Li CX, Liang S, Gao L, et al. Cardiovascular outcomes associated with SGLT-2 inhibitors versus other glucose-lowering drugs in patients with type 2 diabetes: A real-world systematic review and meta-analysis. PLoS One 2021;16:e0244689 [Crossref] [PubMed]
- Birkeland KI, Jørgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol 2017;5:709-17. [Crossref] [PubMed]
- Okunrintemi V, Mishriky BM, Powell JR, et al. Sodium-glucose co-transporter-2 inhibitors and atrial fibrillation in the cardiovascular and renal outcome trials. Diabetes Obes Metab 2021;23:276-80. [Crossref] [PubMed]
- Bonora BM, Raschi E, Avogaro A, et al. SGLT-2 inhibitors and atrial fibrillation in the Food and Drug Administration adverse event reporting system. Cardiovasc Diabetol 2021;20:39. [Crossref] [PubMed]
- Shao Q, Meng L, Lee S, et al. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol 2019;18:165. [Crossref] [PubMed]
- Duran M, Ziyrek M, Alsancak Y. Effects of SGLT2 Inhibitors as an Add-on Therapy to Metformin on Electrocardiographic Indices of Ventricular Repolarization. Acta Cardiol Sin 2020;36:626-32. [PubMed]
- Sato T, Aizawa Y, Yuasa S, et al. The Effect of Dapagliflozin Treatment on Epicardial Adipose Tissue Volume and P-Wave Indices: An Ad-hoc Analysis of The Previous Randomized Clinical Trial. J Atheroscler Thromb 2020;27:1348-58. [Crossref] [PubMed]
- Hatem SN, Sanders P. Epicardial adipose tissue and atrial fibrillation. Cardiovasc Res 2014;102:205-13. [Crossref] [PubMed]
- Bode D, Semmler L, Oeing CU, et al. Implications of SGLT Inhibition on Redox Signalling in Atrial Fibrillation. Int J Mol Sci 2021;22:5937. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


