Advances in the diagnosis of tuberculous pleuritis
Introduction
It is estimated that between 2 and 3 billion people are infected with Mycobacterium tuberculosis (MT) worldwide, of whom 5–15% will develop the tuberculosis (TB) disease during their lifetime (1). In 2014, there were 9.6 million new TB cases, of which 12% were human immunodeficiency virus (HIV)-positive (1). Disease burden was particularly high in South-East Asia and the Western Pacific regions (58% of all cases) (1). Globally, an estimated 3.3% of new cases and 20% of previously treated ones are multidrug-resistant.
Extra-pulmonary sites of infection commonly include the pleura. In Spain, a country with a low incidence of TB (10.8 cases/100,000 population in 2014), pulmonary parenchymal disease accounted for 71%, while lymph node and pleural involvements for 7% and 5%, respectively, of all TB cases (2).
At the author’s institution in Lleida (Spain), TB was reported to be the fourth leading cause of pleural effusion (9% of 3,077 consecutive patients who were subjected to a diagnostic thoracentesis), following cancer, heart failure and pneumonia during the 1994–2013 period (3). However, the rate of TB pleuritis has steadily declined in the region over the years and, in 2015, only 7 (1.9%) of 352 new pleural effusions were caused by it (unpublished observation). In geographical areas with a high TB incidence, it is believed that TB pleural effusions commonly develop from a primary infection, whereas in countries with lower TB incidence they more likely result from a reactivation of a latent infection. HIV was one of the greatest risk factors for developing pleural TB prior to the routine use of effective antiretroviral therapies, but current rates of active TB are likely lower for those patients under the newer regimens.
Pleural TB effusion is thought to occur when a sub-pleural parenchymal lesion ruptures, releasing a small number of tuberculous bacilli into the pleural space, which in turn triggers a local immunological response. A neutrophilic influx takes place, which is followed by monocyte migration and a strong T-helper type 1 lymphocyte reaction (4).
Patients with pleural TB are commonly young males (70%) who present with an acute or subacute syndrome characterized by fever (>80%), cough (75%), pleuritic chest pain (70%) and other potential symptoms (e.g., dyspnea, constitutional symptoms) (4,5). The peripheral leukocyte count is usually normal. Effusions are unilateral in 95% of the cases, occupy half or more of the hemithorax in nearly 50% (5), and coexist with ipsilateral lung parenchymal involvement in 15–27% on chest radiographs (5,6) and up to 85% on computed tomography scans (mainly micronodules and interlobular septal thickening) (7). The tuberculin skin test is negative in approximately 30–40% of cases (8-10).
Provided that more than a minimal amount of fluid exists, all suspected TB effusions should be sampled for chemistry analysis. According to Light’s criteria, pleural fluid is always exudative (5). A lymphocytic predominance (>50% of the total leukocyte count) is seen in about 90% of cases, neutrophilic fluids being more characteristic early in the disease course. Interestingly, when the fluid is neutrophil-rich, the likelihood of isolating MT from pleural fluid or sputum significantly increases (5). About two-thirds of patients exhibit pleural protein concentrations greater than 5 g/dL, while glucose levels <60 mg/dL and pH <7.20 are observed in <25% and <10% of cases, respectively (5).
Limitations of classical diagnostic tests for pleural TB
A definitive diagnosis of TB pleuritis can only be confirmed by the identification of MT, either by microscopy and/or culture, from sputum, pleural fluid or pleural biopsy specimens. Unfortunately, this is accomplished in a relatively low number of cases. In a Spanish series of 1,835 patients with pleural TB recruited during the 2000–2009 period, acid-fast smears (Ziehl-Neelsen) and solid culture media (Lowenstein-Jensen) of sputum samples were positive in 8% and 41%, respectively (6). In the absence of adequate sputum samples, it is imperative to induce sputum by the inhalation of saline. Moreover, pleural fluid smear microscopy and cultures were only positive in 6% and 36%, respectively, of 548 TB patients from another Spanish study (11). The yield from examining for acid-fast bacilli and culturing pleural tissue biopsies was 24% and 53%, respectively (11). In HIV-infected individuals microbiological investigations are more sensitive, presumably due to an impaired bacterial clearance from the pleural space in the context of immunosuppression. For example, in one study the frequency of positive pleural fluid cultures was reported to be 63.6% and 29.5% in 42 HIV-positive and 132 HIV-negative TB patients, respectively (P=0.001) (12).
In addition to the unsatisfactory yield from the preceding classical methods, MT has a long division time and culture of clinical specimens in solid media may take 4–6 weeks to become positive, which precludes immediate clinical decisions and timely treatment.
The observation of caseating granulomas on histological examination of parietal pleural biopsies performed with closed (Abrams or Cope) or cutting-needles (Tru-cut), preferably under ultrasound guidance, has traditionally been a gold diagnostic standard for TB (13). Since TB affects the pleura diffusely, medical thoracoscopy is reserved for the small number of cases where image-guided closed pleural biopsies fail to provide a diagnosis. In one study, blind pleural biopsies demonstrated caseating granulomas in 401 of 517 (77.6%) patients with TB pleurisy (11). The presence of non-caseating granulomas may even be considered diagnostically adequate in high burden settings. Nevertheless, they are not a definitive proof since granulomatous pleuritis may also characterize fungal infections, sarcoidosis, and some vasculitis and rheumatic autoimmune diseases.
Optimizing culture media
Solid media (either egg- or agar-based) have long been the standard for culturing mycobacteria. However, liquid or broth media increases sensitivity and decreases time to positivity compared with solid (14 vs. 21 days) (14). Examples of liquid media include the BacT/ALERT, BACTEC MGIT, VersaTrek and MODS (microscopic observation drug susceptibility). In a study of 382 patients with TB pleurisy (98% HIV-negative) the yield of mycobacterial liquid cultures (BACTEC MGIT) was 63% for pleural fluid, 48% for sputum, and 79% for the combination (15). Some of these findings were further confirmed in a smaller series of 58 pleural TB, in which fluid culture on liquid media had a sensitivity of 60.3% (16). Surprisingly, inoculating just 5 mL instead of 100 mL of pleural fluid did not influence the proportion of positive cultures (50% vs. 53.5%), though it slightly prolonged the time to culture positivity (15.6 vs. 13.7 days) (16). In a former study, MODS was significantly more sensitive than the Lowenstein-Jensen cultures of pleural biopsy specimens from 70 patients with proven TB pleurisy (81% vs. 51%) (17).
Nucleic acid amplification assays
Nucleic acid amplification tests (NAAT), which amplify MT-specific nucleic acid sequences with a nucleic acid probe [polymerase chain reaction (PCR)], enable direct and rapid detection of MT in clinical samples, including pleural fluid and tissue biopsies. Like cultures, they are accepted as confirmatory tests for TB due to their almost absolute specificity. Unfortunately, both in-house and commercialized NAAT (e.g., COBAS Taqman, AMTD) lack enough sensitivity for pleural TB (18), particularly in smear or culture negative specimens. Thus, a negative NAAT should not be used to rule out TB. In a meta-analysis of 14 studies, the pooled sensitivity and specificity of commercially available automated NAAT for identifying pleural TB was found to be 62% and 98%, respectively (19). Major drawbacks of these commercial tests are their high cost and the need for laboratory infrastructure and skilled technicians.
Xpert-MTB/RIF is a rapid, cartridge-based, fully automated real-time PCR test which simultaneously detects MT and rifampin resistant strains in less than two hours with minimal hands-on technical time. A meta-analysis of 24 studies, totaling 2,846 patients, determined the accuracy of Xpert-MTB/RIF on pleural fluid samples for detecting TB pleurisy (20). It was found that the pooled sensitivity and specificity of the test was 51.4% and 98.6% [area under the summary receiver-operating characteristic (SROC) curve 0.843] when a culture was used as the reference standard. However, test sensitivity significantly dropped (22.7%) if a composite reference standard was employed, while specificity remained high (99.8%, area under the SROC curve 0.721). In conclusion, Xpert-MTB/RIF has poor sensitivity and a limited diagnostic capacity, probably because the organism burden is low in pleural fluid. Xpert’s performance might be greater with pleural tissue samples (85.5% sensitivity and 97.2% specificity in a study of 134 patients which used MT culture from pleural biopsy specimens as the reference standard) (21).
Immune-based tests
The interferon-Ƴ release assays (IGRA) are T-cell based tests that measure interferon-Ƴ release by sensitized T-cells in response to MT-specific antigens (e.g., ESAT-6, CFP-10). There are currently two commercially available IGRAs: QuantiFERON®-TB Gold in Tube-test (QFT-GIT) and T-SPOT® TB test (T-Spot). Both blood and pleural fluid samples can be processed for IGRAs, but like tuberculin skin tests, these assays are limited by their inability to differentiate latent from active disease and, consequently, cannot replace appropriate microbiological and molecular investigations. A meta-analysis of 21 studies evaluating the performance of blood- and pleural fluid-based IGRAs (1,085 and 727 patients, respectively) for the diagnosis of pleural TB showed that the pooled sensitivities were 77% and 72%, respectively, and the corresponding specificities were 71% and 78% (22). This poor diagnostic accuracy makes IGRAs unsuitable for diagnostic purposes.
Biomarkers
The low yield of microbiological studies and the invasiveness of pleural biopsies have stimulated the search for TB fluid biomarkers. Although many have been evaluated during the last decades (23), the most qualified is adenosine deaminase (ADA), a predominant T-lymphocyte enzyme.
ADA
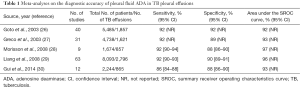
Since first reported in 1978 (24), the measurement of pleural fluid ADA has consistently demonstrated a high accuracy for diagnosing pleural TB (25). Five meta-analyses have shown that pleural fluid ADA has a sensitivity of approximately 92%, a specificity of 90%, and an area under the SROC curve of 0.95 for identifying TB (26-30) (Table 1). Even though the most widely accepted threshold ADA value is 35–40 U/L, some studies have reported that pleural fluid ADA decreases with age, therefore suggesting that lower cutoffs should probably be considered in older patients to reduce the number of false-negative results (31,32).
Full table
Other than TB pleuritis, the main diseases associated with a high pleural fluid ADA level are complicated parapneumonic effusions, empyemas and lymphomas (25). In one study, 44% of non-purulent complicated parapneumonic effusions, 70% of empyemas, and 57% of lymphomas showed pleural ADA levels greater than 35 U/L (25). Fortunately, the former two are relatively easy to differentiate from TB by the clinical picture and the predominance of neutrophils in the pleural fluid. Interestingly, an ADA activity in pleural fluid >250 U/L is highly suggestive of empyema or lymphoma rather than TB (25).
ADA comprises two isoenzymes, namely ADA1 and ADA2. ADA1, an ubiquitous enzyme that may be produced by many different cell types, including neutrophils, explains most false-positive cases in non-TB effusions. In contrast, ADA2 is secreted only by monocytes and macrophages and is the predominant isoenzyme (85%) in TB effusions (5). Although ADA2 slightly increases the sensitivity and specificity of the total ADA in diagnosing TB pleuritis (33), it probably adds little in the majority of cases.
In geographical areas with moderate to high incidence of the disease, ADA has virtually substituted blind pleural biopsies for diagnostic purposes. In regions with low disease burden ADA is still of value in that a low level almost entirely rules out TB (i.e., the chance of an effusion with pleural fluid ADA under 35 U/L being of TB etiology is negligible) (25,34). Despite strong supporting evidence, nearly four decades later there is still some reluctance to accept ADA for expedited clinical decision making. Detractors highlight the inability of the test to provide either drug sensitivity information (which may be paramount only in countries where drug-resistant TB is a concern) or a conclusive demonstration of the infection.
Interferon-Ƴ
Interferon-Ƴ is a pro-inflammatory cytokine which is secreted by Th1 cells, cytotoxic T cells and natural killer cells. It increases the mycobactericidal activity of macrophages. Free, unstimulated interferon-Ƴ levels in pleural fluid, measured by enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay, are valuable in the identification of TB effusions with sensitivity and specificity similar to or, in some studies, slightly better than ADA (35,36). In a meta-analysis of 22 studies, totaling 2,101 patients with pleural effusions (of whom 782 had TB), interferon-Ƴ measurements yielded 89% sensitivity, 97% specificity and area under the SROC curve of 0.99 for the diagnosis of TB (37). High cost and lack of a broadly accepted discriminative cutoff for interferon-Ƴ, still makes ADA the test of choice.
Interferon-Ƴ-induced protein of 10 KDa (IP-10)
IP-10 is a chemokine which is expressed in inflamed tissues by resident cells and monocytes-macrophages. It is involved in trafficking monocytes and activated T-helper type 1 lymphocytes to inflamed foci. The sum of seven series, comprising 629 patients (including 304 with TB pleuritis) in whom pleural fluid IP-10 concentrations were measured by ELISA, shows that the test has a mean sensitivity of 84%, and a specificity of 90% for discriminating between TB and non-TB effusions at varying threshold values (36,38-43). Even though it is a relatively good test, IP-10 does not surpasses the discriminating properties of interferon-Ƴ.
Interleukin-27 (IL-27)
IL-27 is a member of the interleukin-12 family which is secreted by antigen-presenting cells (e.g., macrophages, dendritic cells) and mediates interferon-Ƴ production and T-helper type 1 responses. To date, five studies have reported the diagnostic usefulness of measuring IL-27 levels in pleural fluid samples using ELISA methodologies (44-48). Their sum, totaling 655 non-tuberculous and 228 TB effusions, yields a mean sensitivity and specificity for IL-27 (92.5% and 90%, respectively) similar to that of ADA in the diagnosis of TB. However, no optimal cutoff value has uniformly been accepted, the reported ones ranging from 391 ng/L (48) to 1,007 ng/L (44). In addition, determination of ADA is simpler and cheaper than that of IL-27. Some studies suggest that either the multiplication of IL-27 and ADA values (46,48), or their combination in an “or” rule (45) may reach a sensitivity of 100% for the identification of TB.
Predictive models
Several predictive models which combine various clinical and laboratory parameters to increase diagnostic capabilities for differentiating TB from non-TB effusions (mainly malignant) have been proposed. Most combinations include pleural fluid ADA along with other easily obtained variables, namely patient’s age, temperature, blood leukocyte count, other fluid chemistries (e.g., percentage of lymphocytes, red blood cell count, proteins, classical tumor markers, interferon-Ƴ, IP-10) and pleural fluid cytology (9,49-51). Overall, these models have displayed high diagnostic accuracies [area under the curve (AUC) >0.95]. However, the diagnostic performance of pleural fluid ADA alone is so high that the real value of combining it with other tests is minimal. For instance, in a study of 971 pleural effusions (56% of tuberculous origin), pleural fluid ADA at a threshold value of >44 U/L had a sensitivity, specificity and AUC of 97.6%, 93.1% and 0.979, respectively, for TB, figures which could not be improved by any combination of tests (11).
Conclusions
Although cultures of sputum and pleural fluid or biopsy samples remain the gold standard for diagnosing pleural TB, they lack sensitivity and may take several weeks to become positive when solid media are employed. For this reason, pathologic examination to evaluate for caseating granulomas is still commonly performed, particularly in countries with low TB prevalence. Nevertheless, due to the invasiveness of pleural biopsies, alternative tests have long been sought after. Among them, pleural fluid ADA is considered a hallmark of TB. In moderate to high burden settings, the finding of a lymphocytic predominant exudate with high ADA levels infers the diagnosis of TB and justifies the prompt initiation of anti-tuberculous therapy (52). Conversely, in areas with low TB prevalence, the high negative predictive value of ADA makes it an excellent rule out test. Even though other fluid biomarkers, such as interferon-Ƴ and IL-27, are also very accurate for labeling pleural TB, they have not attained practical applicability nor proven to be superior to the widely accepted ADA. Given the critical importance of microbiology for a definitive TB diagnosis, spontaneous or induced sputum, as well as pleural fluid samples, should be cultured on both a solid and a liquid medium.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- World Health Organization. 2015 Global tuberculosis report. Available online: https://www.health-e.org.za/wp-content/uploads/2015/10/Global-TB-Report-2015-FINAL-2.pdf
- Informe epidemiológico sobre la situación de la tuberculosis en España. Año 2014. Centro Nacional de Epidemiología. Instituto de Salud Carlos III. Available online: http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/pdf_2015/TB_Informe_2014.pdf
- Porcel JM, Esquerda A, Vives M, et al. Etiology of pleural effusions: analysis of more than 3,000 consecutive thoracenteses. Arch Bronconeumol 2014;50:161-5. [PubMed]
- Porcel JM. Tuberculous pleural effusion. Lung 2009;187:263-70. [Crossref] [PubMed]
- Bielsa S, Palma R, Pardina M, et al. Comparison of polymorphonuclear- and lymphocyte-rich tuberculous pleural effusions. Int J Tuberc Lung Dis 2013;17:85-9. [Crossref] [PubMed]
- Valdés L, Ferreiro L, Cruz-Ferro E, et al. Recent epidemiological trends in tuberculous pleural effusion in Galicia, Spain. Eur J Intern Med 2012;23:727-32. [Crossref] [PubMed]
- Ko JM, Park HJ, Kim CH. Pulmonary changes of pleural TB: up-to-date CT imaging. Chest 2014;146:1604-11. [Crossref] [PubMed]
- Valdés L, Alvarez D, San José E, et al. Tuberculous pleurisy: a study of 254 patients. Arch Intern Med 1998;158:2017-21. [Crossref] [PubMed]
- Porcel JM, Vives M. Differentiating tuberculous from malignant pleural effusions: a scoring model. Med Sci Monit 2003;9:CR175-80. [PubMed]
- Baumann MH, Nolan R, Petrini M, et al. Pleural tuberculosis in the United States: incidence and drug resistance. Chest 2007;131:1125-32. [Crossref] [PubMed]
- Sahn SA, Huggins JT, San José ME, et al. Can tuberculous pleural effusions be diagnosed by pleural fluid analysis alone? Int J Tuberc Lung Dis 2013;17:787-93. [Crossref] [PubMed]
- Marjani M, Yousefzadeh A, Baghaei P, et al. Impact of HIV infection on tuberculous pleural effusion. Int J STD AIDS 2016;27:363-9. [Crossref] [PubMed]
- Bibby AC, Maskell NA. Pleural biopsies in undiagnosed pleural effusions; Abrams vs image-guided vs thoracoscopic biopsies. Curr Opin Pulm Med 2016;22:392-8. [Crossref] [PubMed]
- Rageade F, Picot N, Blanc-Michaud A, et al. Performance of solid and liquid culture media for the detection of Mycobacterium tuberculosis in clinical materials: meta-analysis of recent studies. Eur J Clin Microbiol Infect Dis 2014;33:867-70. [Crossref] [PubMed]
- Ruan SY, Chuang YC, Wang JY, et al. Revisiting tuberculous pleurisy: pleural fluid characteristics and diagnostic yield of mycobacterial culture in an endemic area. Thorax 2012;67:822-7. [Crossref] [PubMed]
- von Groote-Bidlingmaier F, Koegelenberg CF, Bolliger CT, et al. The yield of different pleural fluid volumes for Mycobacterium tuberculosis culture. Thorax 2013;68:290-1. [Crossref] [PubMed]
- Tovar M, Siedner MJ, Gilman RH, et al. Improved diagnosis of pleural tuberculosis using the microscopic- observation drug-susceptibility technique. Clin Infect Dis 2008;46:909-12. [Crossref] [PubMed]
- Trajman A, da Silva Santos Kleiz de Oliveira EF, Bastos ML, et al. Accuracy of polimerase chain reaction for the diagnosis of pleural tuberculosis. Respir Med 2014;108:918-23. [Crossref] [PubMed]
- Pai M, Flores LL, Hubbard A, et al. Nucleic acid amplification tests in the diagnosis of tuberculous pleuritis: a systematic review and meta-analysis. BMC Infect Dis 2004;4:6. [Crossref] [PubMed]
- Sehgal IS, Dhooria S, Aggarwal AN, et al. Diagnostic Performance of Xpert MTB/RIF in Tuberculous Pleural Effusion: Systematic Review and Meta-analysis. J Clin Microbiol 2016;54:1133-6. [Crossref] [PubMed]
- Du J, Huang Z, Luo Q, et al. Rapid diagnosis of pleural tuberculosis by Xpert MTB/RIF assay using pleural biopsy and pleural fluid specimens. J Res Med Sci 2015;20:26-31. [PubMed]
- Aggarwal AN, Agarwal R, Gupta D, et al. Interferon Gamma Release Assays for Diagnosis of Pleural Tuberculosis: a Systematic Review and Meta-Analysis. J Clin Microbiol 2015;53:2451-9. [Crossref] [PubMed]
- Skouras VS, Kalomenidis I. Pleural fluid tests to diagnose tuberculous pleuritis. Curr Opin Pulm Med 2016;22:367-77. [Crossref] [PubMed]
- Piras MA, Gakis C, Budroni M, Andreoni G. Adenosine deaminase activity in pleural effusions: an aid to differential diagnosis. Br Med J 1978;2:1751-2. [Crossref] [PubMed]
- Porcel JM, Esquerda A, Bielsa S. Diagnostic performance of adenosine deaminase activity in pleural fluid: a single-center experience with over 2100 consecutive patients. Eur J Intern Med 2010;21:419-23. [Crossref] [PubMed]
- Goto M, Noguchi Y, Koyama H, et al. Diagnostic value of adenosine deaminase in tuberculous pleural effusion: a meta-analysis. Ann Clin Biochem 2003;40:374-81. [Crossref] [PubMed]
- Greco S, Girardi E, Masciangelo R, et al. Adenosine deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: a meta-analysis. Int J Tuberc Lung Dis 2003;7:777-86. [PubMed]
- Morisson P, Neves DD. Evaluation of adenosine deaminase in the diagnosis of pleural tuberculosis: a Brazilian meta-analysis. J Bras Pneumol 2008;34:217-24. [Crossref] [PubMed]
- Liang QL, Shi HZ, Wang K, et al. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med 2008;102:744-54. [Crossref] [PubMed]
- Gui X, Xiao H. Diagnosis of tuberculosis pleurisy with adenosine deaminase (ADA): a systematic review and meta-analysis. Int J Clin Exp Med 2014;7:3126-35. [PubMed]
- Abrao FC, de Abreu IR, Miyake DH, et al. Role of adenosine deaminase and the influence of age on the diagnosis of pleural tuberculosis. Int J Tuberc Lung Dis 2014;18:1363-9. [Crossref] [PubMed]
- Lee SJ, Kim HS, Lee SH, et al. Factors influencing pleural adenosine deaminase level in patients with tuberculous pleurisy. Am J Med Sci 2014;348:362-5. [Crossref] [PubMed]
- Zemlin AE, Burgess LJ, Carstens ME. The diagnostic utility of adenosine deaminase isoenzymes in tuberculous pleural effusions. Int J Tuberc Lung Dis 2009;13:214-20. [PubMed]
- Arnold DT, Bhatnagar R, Fairbanks LD, et al. Pleural fluid adenosine deaminase (pfADA) in the diagnosis of tuberculous effusions in a low incidence population. PLoS One 2015;10:e0113047. [Crossref] [PubMed]
- Li M, Wang H, Wang X, et al. Diagnostic accuracy of tumor necrosis factor-alpha, interferon-gamma, interleukin-10 and adenosine deaminase 2 in differential diagnosis between tuberculous pleural effusion and malignant pleural effusion. J Cardiothorac Surg 2014;9:118. [Crossref] [PubMed]
- Klimiuk J, Krenke R, Safianowska A, et al. Diagnostic performance of different pleural fluid biomarkers in tuberculous pleurisy. Adv Exp Med Biol 2015;852:21-30. [Crossref] [PubMed]
- Jiang J, Shi HZ, Liang QL, et al. Diagnostic value of interferon-gamma in tuberculous pleurisy: a metaanalysis. Chest 2007;131:1133-41. [Crossref] [PubMed]
- Okamoto M, Kawabe T, Iwasaki Y, et al. Evaluation of interferon-gamma, interferon-gamma-inducing cytokines, and interferon-gamma-inducible chemokines in tuberculous pleural effusions. J Lab Clin Med 2005;145:88-93. [Crossref] [PubMed]
- Supriya P, Chandrasekaran P, Das SD. Diagnostic utility of interferon-gamma-induced protein of 10 kDa (IP-10) in tuberculous pleurisy. Diagn Microbiol Infect Dis 2008;62:186-92. [Crossref] [PubMed]
- Dheda K, Van-Zyl Smit RN, Sechi LA, et al. Clinical diagnostic utility of IP-10 and LAM antigen levels for the diagnosis of tuberculous pleural effusions in a high burden setting. PLoS One 2009;4:e4689. [Crossref] [PubMed]
- Sutherland JS, Garba D, Fombah AE, et al. Highly accurate diagnosis of pleural tuberculosis by immunological analysis of the pleural effusion. PLoS One 2012;7:e30324. [Crossref] [PubMed]
- Wang H, Yue J, Yang J, et al. Clinical diagnostic utility of adenosine deaminase, interferon-γ, interferon-γ-induced protein of 10 kDa, and dipeptidyl peptidase 4 levels in tuberculous pleural effusions. Heart Lung 2012;41:70-5. [Crossref] [PubMed]
- Yang Q, Cai Y, Zhao W, et al. IP-10 and MIG are compartmentalized at the site of disease during pleural and meningeal tuberculosis and are decreased after antituberculosis treatment. Clin Vaccine Immunol 2014;21:1635-44. [Crossref] [PubMed]
- Yang WB, Liang QL, Ye ZJ, et al. Cell origins and diagnostic accuracy of interleukin 27 in pleural effusions. PLoS One 2012;7:e40450. [Crossref] [PubMed]
- Wu YB, Ye ZJ, Qin SM, et al. Combined detections of interleukin 27, interferon-γ, and adenosine deaminase in pleural effusion for diagnosis of tuberculous pleurisy. Chin Med J (Engl) 2013;126:3215-21. [PubMed]
- Valdés L, San José E, Ferreiro L, et al. Interleukin 27 could be useful in the diagnosis of tuberculous pleural effusions. Respir Care 2014;59:399-405. [Crossref] [PubMed]
- Sun M, Yan D, Jiang S, et al. Diagnostic value of interleukin-27 in tuberculous pleural effusion. Zhonghua Yi Xue Za Zhi 2014;94:2641-4. [PubMed]
- Skouras VS, Magkouta SF, Psallidas I, et al. Interleukin-27 improves the ability of adenosine deaminase to rule out tuberculous pleural effusion regardless of pleural tuberculosis prevalence. Infect Dis (Lond) 2015;47:477-83. [Crossref] [PubMed]
- Valdés L, San José ME, Pose A, et al. Diagnosing tuberculous pleural effusion using clinical data and pleural fluid analysis A study of patients less than 40 years-old in an area with a high incidence of tuberculosis. Respir Med 2010;104:1211-7. [Crossref] [PubMed]
- Valdés L, San-José E, Ferreiro L, et al. Predicting malignant and tuberculous pleural effusions through demographics and pleural fluid analysis of patients. Clin Respir J 2015;9:203-13. [Crossref] [PubMed]
- Klimiuk J, Safianowska A, Chazan R, et al. Development and Evaluation of the New Predictive Models in Tuberculous Pleuritis. Adv Exp Med Biol 2015;873:53-63. [Crossref] [PubMed]
- Porcel JM, Azzopardi M, Koegelenberg CF, et al. The diagnosis of pleural effusions. Expert Rev Respir Med 2015;9:801-15. [Crossref] [PubMed]


