Progression of coronary artery calcification at the crossroads: sign of progression or stabilization of coronary atherosclerosis?
Introduction
Coronary artery calcification (CAC) has been strongly established as an independent predictor of adverse events, with a significant incremental prognostic value over traditional risk stratification algorithms (1-3). Asymptomatic and even symptomatic patients with absence of calcifications (CAC zero) assessed by cardiac computed tomography have a very low incidence of events at long-term follow-up (1). Furthermore, CAC progression has been associated with higher rates of events (4).
Routine medical therapy for coronary artery disease (CAD) aims to slow the progression of atherosclerosis. Indeed, a vast number of randomized studies and meta-analysis have shown the effectiveness of statins in secondary prevention, not only by providing a significant reduction in coronary events, but also in their ability to slow progression and even promote plaque regression (5-7). However, evidence regarding the effect of routine medical therapy on CAC has yielded conflicting results, with initial studies showing significant CAC regression, and contemporaneous data showing rather the opposite (8-12). Furthermore, complementary prescription of comprehensive lifestyle modification on top of contemporary secondary prevention strategies in patients with CAD has no impact on CAC progression but significant benefit for blood pressure, heart rate and the need of anti-ischemic medication (13).
Accordingly, there is currently a great controversy on whether progression of CAC is a sign of progression or stabilization of CAD.
CAC scoring by computed tomography: Etiology and prognostic value
CAC is a hallmark of atherosclerosis, and is highly related to increasing age (14). Since life expectancy has significantly improved in the past decades, it is of utmost importance to refine the role of calcium as a prognostic marker. Both ex vivo and intravascular ultrasound (IVUS) studies have shown that CAC is closely related to atherosclerotic plaque burden, although the molecular basis of this process remains uncertain (15-17). Recent molecular imaging studies support the current notion that vascular calcification is not a passive degenerative process, but actually an active process that leads to ectopic mineralization promoted by the expression of multiple pro-osteogenic cytokines, transcription factors, and mineralization-regulating proteins by macrophages and other inflammatory cells (18). In this regard, fluorescence molecular multimodality imaging has shown potential to provide insightful data concerning arterial osteogenesis at much earlier stages of atherosclerosis (19,20).
In one of the first studies addressing the association between CAC and plaque burden in post mortem specimens, Sangiorgi et al. showed a significant correlation between calcium and plaque areas, being this significant both on a per heart and a per vessel basis (17). Nonetheless, the extent of CAC is not strongly related to the degree of luminal stenosis on a per lesion basis (17,21). Indeed, despite CAC is commonly associated to advanced stages of atherosclerosis and to a more stable and quiescent phenotype, calcifications can be present in early stages of CAD, as discussed above. Besides, most thin-cap fibroatheroma lesions, the main substrate of plaque rupture, show microcalcifications within the necrotic core or at the periphery (14). In addition, studies using advanced imaging including micro-CT and optical coherence tomography (OCT) have related microcalcifications within the thin fibrous cap to vulnerable features, and to an increased risk of plaque disruption (22,23).
Before the widespread installation of multidetector computed tomography (MDCT), early studies using electron beam CT established CAC as an independent predictor of events with an incremental prognostic value over traditional risk stratification algorithms (2,24).
CAC assessment by MDCT is a simple procedure that does not require contrast administration or heart rate lowering medication. CAC has high sensitivity and negative predictive value for the detection of obstructive CAD. Indeed, not only the absence of coronary calcifications (CAC zero) has shown a nearly 100% sensitivity and negative predictive value to rule out obstructive CAD, as discussed above, but also a CAC >400 has shown modest specificity and positive predictive value to identify obstructive CAD (1,25,26). A number of studies have explored the relationship between CAC scoring and myocardial perfusion imaging (MPI). Among them, one study including low risk patients showed that only 2% of patients with CAC <100 have abnormal MPI studies, compared to 31% of patients with CAC >400 (27,28). A CAC score ≥709 has been suggested as the optimal cutoff for detecting CAD missed by SPECT imaging, improving the sensitivity of SPECT from 76% to 86% (29).
Furthermore, CAC scoring is associated to a very low effective radiation dose (~1.0 mSv) and it has been extensively validated as an independent predictor of major adverse cardiac events and total mortality in asymptomatic patients, providing a significant incremental value over traditional risk factors and functional studies (30-33).
Overall, the robust evidence available has led to the inclusion of CAC in a number of guidelines for risk stratification of asymptomatic intermediate risk patients (34,35).
A number of absolute CAC score thresholds have been defined for risk prediction ranging from very low risk to very high risk of events (CAC 0; 1–99; 100–399; 400–999; and ≥1,000), being asymptomatic individuals with CAC >400 at a similar risk of events than patients with established CAD (31-33). Nevertheless, the close relationship between CAC and age mandates an assessment according to age and sex (Figure 1). In fact, Becker et al. have shown that CAC above the 75th percentile is associated with significantly higher rates of cardiovascular death and myocardial infarction than patients with CAC scores below the 75th percentile (36).
Asymptomatic and even symptomatic patients with absence of calcifications (CAC zero) assessed by MDCT have a very low incidence of events at long-term follow-up (3,17). Of note, a large body of evidence renders the absence of calcification a 5-year safety window, with a 0.10% annual risk of events (2,32,37-41).
Notwithstanding, the absence of calcium does not rule out the presence of plaque. Indeed, CAC zero in symptomatic patients should lead to a cautious interpretation due to a number of factors. Firstly, approximately 30% of acute coronary thromboses, particularly in young women and in smokers, are attributed to plaque erosion, a type of plaque that has no lipid core or calcifications and is therefore so far undetectable to any invasive and non-invasive technique, although recent studies suggested that they might be detected by OCT (42,43). Secondly, spotty calcifications might be occasionally undetected by the 3 mm slices that are routinely used for CAC assessment by MDCT. Spotty calcifications can be more easily identified using either catheter-based techniques (IVUS and OCT) or non-invasive imaging using MDCT coronary angiography. This feature (defined by IVUS as lesions 1 to 4 mm in length containing an arc of calcification of <90°, and <3 mm by MDCT) is commonly observed in culprit lesions of patients with acute coronary syndromes, although it has a low positive predictive value for the prediction of events compared to other high risk findings such as positive remodeling or low attenuation plaques (44,45). Besides, the size of the aforementioned microcalcifications within the thin fibrous cap (<65 µm) precludes non-invasive detection by means of MDCT and even by IVUS.
Other worth mentioning lesions are calcified nodules. These protrusive superficial lesions, although very infrequent, have been related to plaque rupture and acute coronary thrombosis, and MDCT might be able to identify them (43,46).
Meaning of CAC progression
Progression of CAD is undoubtedly related to adverse clinical outcomes. Based on the robust evidence confirming the role of CAC as an independent predictor of death and myocardial infarction, and the fact that CAC is closely associated to the extent of CAD; it might be assumed that CAC progression would also portent a worse prognosis (32,47,48).
This might potentially be related to the fact that spotty calcifications are associated with a larger atherosclerotic burden and to accelerated plaque progression despite use of secondary prevention strategies (49).
Nonetheless, evidence in this regard is inconclusive and the clinical significance of CAC progression remains to be established. In a consecutive series of 4,609 asymptomatic individuals who underwent serial scanning, Budoff et al. found that CAC progression (defined as difference between square root of baseline and square root of follow-up CAC score >2.5; or >15% yearly increase) added a significant incremental value over baseline CAC, time between scans, and demographical characteristics in predicting all-cause mortality (4).
More recently, a subanalysis of the Multi-Ethnic Study of Atherosclerosis (MESA) study showed a linear relationship between CAC progression and risk of cardiovascular events, and identified a three- to six-fold increased rate of events in those with an annual progression ≥300 units (50). CAC progression has been related to several traditional modifiable and non-modifiable cardiovascular risk factors, as well as to novel risk factors such as C-reactive protein, cystatin-C, and to low adiponectin levels (51-54). However, there are no conclusive findings regarding their specific predictive value.
Routine medical therapy for CAD aims to slow the progression of atherosclerosis. Conventional and novel, predominantly lipid lowering, pharmacological strategies have attempted to achieve plaque stabilization and even shown to promote plaque regression (7,55,56). More in particular, aggressive lipid-lowering with high-dose statins has overall accomplished this goal, as assessed by several IVUS studies (6,7,57).
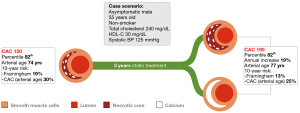
Nonetheless, the significance of CAC alterations with regard to the underlying shift in plaque volume remains unknown. CAC progression might be attributed to plaque progression into a more unstable phenotype with accumulation of microcalcifications, and this would be justified by the aforementioned evidence supporting the deleterious role of CAC progression. However, CAC progression in the context of lifestyle modification and lipid lowering therapies might also be related to a shift towards a more stable phenotype. This paradox is portrayed in Figure 1. In other words: (I) does CAC progression mean plaque progression or plaque stabilization?
Effect of statins on CAC
Lipid lowering therapies showed a strikingly improved clinical outcome of patients with CAD, both in the primary and secondary prevention realms (58,59). Reversal of coronary atherosclerosis with intensive statin therapy has been reported in both the peripheral and coronary circulation using diverse invasive and non-invasive imaging tools (6,7,60-64). Indeed, a recent study using IVUS radiofrequency data (RF) analysis demonstrated that in patients with ST-elevation acute myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention, high-dose rosuvastatin therapy over 13 months leads to regression of coronary atherosclerosis in non culprit vessels. In this study, 74% of patients showed regression in at least one non-culprit vessel. Of note, plaque regression could not be attributed to changes in the necrotic core extent or in the number of IVUS-derived thin cap fibroatheromas (65).
Similarly, the long term results of the Study of coronary Atheroma by inTravascular Ultrasound: the effect of Rosuvastatin vs. atorvastatiN (SATURN) demonstrated that high-dose statin therapy promoted a significant regression in percent atheroma volume, despite both the necrotic core volume and the frequency of fibroatheromas remained stable. Of note, a significant increase in dense calcium volume was reported in this study (57).
Accordingly, the mechanisms involved in plaque stabilization and regression are still not fully understood, being so far ascribed to changes in LDL-C and HDL-C (60,64,66).
The observed plaque stabilization effect induced by statins might potentially be attributed to pleomorphic effect including a decrease in the lipid content of plaques, a reduction in the inflammatory burden and an improvement in endothelial function; all promoting a more stable phenotype (67-71). Notwithstanding, most evidence in this regard remains inconclusive or speculative.
CAC was conceived as a non-invasive imaging tool aimed at non-invasive assessment of CAD (72). Later on, it has been proposed as a useful tool to monitor the impact of diverse medical therapies on atherosclerosis (8).
Evidence regarding the effect of routine medical therapy on CAC has yielded conflicting results, with initial studies showing significant CAC regression, and contemporaneous data showing rather the opposite (8-10,73).
Indeed, a study published in the New England of Medicine in 1998 reported a significant reduction in coronary artery calcium volume assessed using electron-beam CT, proposing a new surrogate endpoint for future prospective clinical studies exploring the effect of drug therapies on CAD (8). The study of Callister et al. promoted the idea that CAC regression could be used as an appealing imaging endpoint of both primary and secondary prevention strategies (74-76).
Nonetheless, all the randomized controlled clinical trials performed have consistently reported a persistent CAC progression despite intensive lipid-lowering treatment (Table 1) (10,77-80). In fact, a recent meta-analysis that included 8 pooled clinical trials evaluating the effect of high intensity, low intensity or no statin on the percent atheroma volume as assessed by IVUS, showed that aggressive statin therapy induced a significant reduction in percent atheroma volume. Of note, high intensity treatment with statins also promoted a significant increase in calcium index compared to the other two groups (11). Indeed, a recent meta-analysis showed continuing progression of coronary calcification despite treatment with statins (12).

Full table
Therefore, in brief: (I) CAC progression is an independent predictor of events; (II) statins promote plaque regression; and (III) statins promote CAC progression.
These paradoxical results are puzzling and warrant the conduction of further studies aimed at the pathophysiological and clinical discrimination between plaque volume progression and CAC progression.
One of the potential explanations might be that conventional reading of CAC studies does not make a distinction between spotty calcifications and dense calcium.
Future discrimination between these two completely different sources of coronary calcium might become a major breakthrough in CAC imaging, since spotty calcifications have been recognized as a marker of high risk plaques (81,82).
Regarding future alternative therapeutic approaches, the combined application of molecular imaging agents with anticalcification drugs such as bisphosphonate might potentially enable targeting at different stages of the disease (18).
Future perspectives
Should the Agatston score be revisited? CAC progression seems inevitable and predictable, with limited influence of cardiovascular risk factors (83).
The unquestioned clinical benefit of statins observed in secondary prevention surpasses the expected benefit based on their lipid lowering effect, being this at least in part explained by their supposed ability to decrease the lipid and macrophage content and to increase the fibrous cap thickness in atherosclerotic plaques, promoting a shift into more stable, calcified lesions (69,84,85).
As it was recently postulated by Shaw et al., CAC predicts risk via an intrinsic property or by being a marker of coexisting high-risk plaques in a stabilization process commanded by coronary artery mineralization (86)? Accordingly, based on the available conflicting evidence, it remains unknown whether CAC progression as a single endpoint can discriminate between two opposite outcomes such as plaque stabilization and atherosclerotic plaque progression (Figure 1). As discussed above, conventional CAC scoring comprises the quantification of coronary calcifications both on per vessel and per patient basis, leading to robust risk stratification of asymptomatic patients as a once-only study. Future developments of the technique warrant the conception of second-generation CAC capable to discriminate between different calcification patterns and spatial distribution, possibly leading to a refinement of the prognostic value and to the application of the technique to longitudinal studies (46).
Until then, the usefulness of CAC once the patient is under statin treatment should be limited. Indeed, a number of medical therapies and even supplementations often used for the management of patients with hypertension or related complications such as atrial fibrillation have shown a significant association to CAC and/or plaque stabilization (55,87,88). The finding of inexorable CAC progression despite the implementation of intensive contemporaneous medical therapy might suggest that further understanding of this phenomenon should be undertaken before the implementation of CAC as a surrogate endpoint for longitudinal studies, or for prospective follow-up of patients under routine medical treatment (9,12,78).
Acknowledgements
None.
Footnote
Conflicts of Interest: We declare that Dr. Patricia Carrascosa is Consultant of GE. The other authors have no conflicts of interest to declare.
References
- Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging 2009;2:692-700. [Crossref] [PubMed]
- Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. New Engl J Med 2008;358:1336-45. [Crossref] [PubMed]
- Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging 2009;2:675-88. [Crossref] [PubMed]
- Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010;3:1229-36. [Crossref] [PubMed]
- Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. New Engl J Med 1998;339:1349-57. [Crossref] [PubMed]
- Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006;295:1556-65. [Crossref] [PubMed]
- Rodriguez-Granillo GA, Agostoni P, Garcia-Garcia HM, et al. Meta-analysis of the studies assessing temporal changes in coronary plaque volume using intravascular ultrasound. Am J Cardiol 2007;99:5-10. [Crossref] [PubMed]
- Callister TQ, Raggi P, Cooil B, et al. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. New Engl J Med 1998;339:1972-8. [Crossref] [PubMed]
- McCullough PA, Chinnaiyan KM. Annual progression of coronary calcification in trials of preventive therapies: a systematic review. Arch Intern Med 2009;169:2064-70. [Crossref] [PubMed]
- Houslay ES, Cowell SJ, Prescott RJ, et al. Progressive coronary calcification despite intensive lipid-lowering treatment: a randomised controlled trial. Heart 2006;92:1207-12. [Crossref] [PubMed]
- Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol 2015;65:1273-82. [Crossref] [PubMed]
- Henein MY, Owen A. Statins moderate coronary stenoses but not coronary calcification: results from meta-analyses. Int J Cardiol 2011;153:31-5. [Crossref] [PubMed]
- Lehmann N, Paul A, Moebus S, et al. Effects of lifestyle modification on coronary artery calcium progression and prognostic factors in coronary patients--3-year results of the randomized SAFE-LIFE trial. Atherosclerosis 2011;219:630-6. [Crossref] [PubMed]
- Virmani R, Kolodgie FD, Burke AP, et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262-75. [Crossref] [PubMed]
- Mintz GS, Pichard AD, Popma JJ, et al. Determinants and correlates of target lesion calcium in coronary artery disease: a clinical, angiographic and intravascular ultrasound study. J Am Coll Cardiol 1997;29:268-74. [Crossref] [PubMed]
- Pundziute G, Schuijf JD, Jukema JW, et al. Head-to-head comparison of coronary plaque evaluation between multislice computed tomography and intravascular ultrasound radiofrequency data analysis. JACC Cardiovasc Interv 2008;1:176-82. [Crossref] [PubMed]
- Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 1998;31:126-33. [Crossref] [PubMed]
- New SE, Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res 2011;108:1381-91. [Crossref] [PubMed]
- Aikawa E, Nahrendorf M, Sosnovik D, et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation 2007;115:377-86. [Crossref] [PubMed]
- Aikawa E, Nahrendorf M, Figueiredo JL, et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 2007;116:2841-50. [Crossref] [PubMed]
- Simons DB, Schwartz RS, Edwards WD, et al. Noninvasive definition of anatomic coronary artery disease by ultrafast computed tomographic scanning: a quantitative pathologic comparison study. J Am Coll Cardiol 1992;20:1118-26. [Crossref] [PubMed]
- Vengrenyuk Y, Cardoso L, Weinbaum S. Micro-CT based analysis of a new paradigm for vulnerable plaque rupture: cellular microcalcifications in fibrous caps. Mol Cell Biomech 2008;5:37-47. [PubMed]
- Kataoka Y, Puri R, Hammadah M, et al. Spotty calcification and plaque vulnerability in vivo: frequency-domain optical coherence tomography analysis. Cardiovasc Diagn Ther 2014;4:460-9. [PubMed]
- Detrano R, Hsiai T, Wang S, et al. Prognostic value of coronary calcification and angiographic stenoses in patients undergoing coronary angiography. J Am Coll Cardiol 1996;27:285-90. [Crossref] [PubMed]
- Budoff MJ, Diamond GA, Raggi P, et al. Continuous probabilistic prediction of angiographically significant coronary artery disease using electron beam tomography. Circulation 2002;105:1791-6. [Crossref] [PubMed]
- Meyer M, Henzler T, Fink C, et al. Impact of coronary calcium score on the prevalence of coronary artery stenosis on dual source CT coronary angiography in caucasian patients with an intermediate risk. Acad Radiol 2012;19:1316-23. [Crossref] [PubMed]
- Chang SM, Nabi F, Xu J, et al. The coronary artery calcium score and stress myocardial perfusion imaging provide independent and complementary prediction of cardiac risk. J Am Coll Cardiol 2009;54:1872-82. [Crossref] [PubMed]
- Berman DS, Wong ND, Gransar H, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol 2004;44:923-30. [Crossref] [PubMed]
- Schepis T, Gaemperli O, Koepfli P, et al. Added value of coronary artery calcium score as an adjunct to gated SPECT for the evaluation of coronary artery disease in an intermediate-risk population. J Nucl Med 2007;48:1424-30. [Crossref] [PubMed]
- Erbel R, Mohlenkamp S, Moebus S, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010;56:1397-406. [Crossref] [PubMed]
- Greenland P, LaBree L, Azen SP, et al. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 2004;291:210-5. [Crossref] [PubMed]
- Arad Y, Goodman KJ, Roth M, et al. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol 2005;46:158-65. [Crossref] [PubMed]
- Vliegenthart R, Oudkerk M, Hofman A, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation 2005;112:572-7. [Crossref] [PubMed]
- Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2007;49:378-402. [Crossref] [PubMed]
- Naghavi M, Falk E, Hecht HS, et al. The first SHAPE (Screening for Heart Attack Prevention and Education) guideline. Crit Pathw Cardiol 2006;5:187-90. [Crossref] [PubMed]
- Becker A, Leber A, Becker C, et al. Predictive value of coronary calcifications for future cardiac events in asymptomatic individuals. Am Heart J 2008;155:154-60. [Crossref] [PubMed]
- Taylor AJ, Bindeman J, Feuerstein I, et al. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol 2005;46:807-14. [Crossref] [PubMed]
- Raggi P, Cooil B, Callister TQ. Use of electron beam tomography data to develop models for prediction of hard coronary events. Am Heart J 2001;141:375-82. [Crossref] [PubMed]
- Shaw LJ, Raggi P, Schisterman E, et al. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 2003;228:826-33. [Crossref] [PubMed]
- LaMonte MJ, FitzGerald SJ, Church TS, et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol 2005;162:421-9. [Crossref] [PubMed]
- Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007;49:1860-70. [Crossref] [PubMed]
- Jia H, Abtahian F, Aguirre AD, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol 2013;62:1748-58. [Crossref] [PubMed]
- Higuma T, Soeda T, Abe N, et al. A Combined Optical Coherence Tomography and Intravascular Ultrasound Study on Plaque Rupture, Plaque Erosion, and Calcified Nodule in Patients With ST-Segment Elevation Myocardial Infarction: Incidence, Morphologic Characteristics, and Outcomes After Percutaneous Coronary Intervention. JACC Cardiovasc Interv 2015;8:1166-76. [Crossref] [PubMed]
- Motoyama S, Ito H, Sarai M, et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J Am Coll Cardiol 2015;66:337-46. [Crossref] [PubMed]
- Nakazato R, Otake H, Konishi A, et al. Atherosclerotic plaque characterization by CT angiography for identification of high-risk coronary artery lesions: a comparison to optical coherence tomography. Eur Heart J Cardiovasc Imaging 2015;16:373-9. [Crossref] [PubMed]
- Thilo C, Gebregziabher M, Mayer FB, et al. Correlation of regional distribution and morphological pattern of calcification at CT coronary artery calcium scoring with non-calcified plaque formation and stenosis. Eur Radiol 2010;20:855-61. [Crossref] [PubMed]
- Raggi P, Cooil B, Shaw LJ, et al. Progression of coronary calcium on serial electron beam tomographic scanning is greater in patients with future myocardial infarction. Am J Cardiol 2003;92:827-9. [Crossref] [PubMed]
- Kiramijyan S, Ahmadi N, Isma'eel H, et al. Impact of coronary artery calcium progression and statin therapy on clinical outcome in subjects with and without diabetes mellitus. Am J Cardiol 2013;111:356-61. [Crossref] [PubMed]
- Kataoka Y, Wolski K, Uno K, et al. Spotty calcification as a marker of accelerated progression of coronary atherosclerosis: insights from serial intravascular ultrasound. J Am Coll Cardiol 2012;59:1592-7. [Crossref] [PubMed]
- Budoff MJ, Young R, Lopez VA, et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013;61:1231-9. [Crossref] [PubMed]
- McEvoy JW, Blaha MJ, Defilippis AP, et al. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol 2010;56:1613-22. [Crossref] [PubMed]
- Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2007;115:2722-30. [Crossref] [PubMed]
- Maahs DM, Ogden LG, Kinney GL, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation 2005;111:747-53. [Crossref] [PubMed]
- Maahs DM, Ogden LG, Kretowski A, et al. Serum cystatin C predicts progression of subclinical coronary atherosclerosis in individuals with type 1 diabetes. Diabetes 2007;56:2774-9. [Crossref] [PubMed]
- Rodriguez-Granillo GA, de Winter S, Bruining N, et al. Effect of perindopril on coronary remodelling: insights from a multicentre, randomized study. Eur Heart J 2007;28:2326-31. [Crossref] [PubMed]
- Rodriguez-Granillo GA, Vos J, Bruining N, Garcia-Garcia HM, de Winter S, Ligthart JM, et al. Long-term effect of perindopril on coronary atherosclerosis progression (from the perindopril's prospective effect on coronary atherosclerosis by angiography and intravascular ultrasound evaluation [PERSPECTIVE] study). Am J Cardiol 2007;100:159-63. [Crossref] [PubMed]
- Puri R, Libby P, Nissen SE, et al. Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. Eur Heart J Cardiovasc Imaging 2014;15:380-8. [Crossref] [PubMed]
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-9. [PubMed]
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279:1615-22. [Crossref] [PubMed]
- Lima JA, Desai MY, Steen H, et al. Statin-induced cholesterol lowering and plaque regression after 6 months of magnetic resonance imaging-monitored therapy. Circulation 2004;110:2336-41. [Crossref] [PubMed]
- Corti R, Fuster V, Fayad ZA, et al. Effects of aggressive versus conventional lipid-lowering therapy by simvastatin on human atherosclerotic lesions: a prospective, randomized, double-blind trial with high-resolution magnetic resonance imaging. J Am Coll Cardiol 2005;46:106-12. [Crossref] [PubMed]
- Yonemura A, Momiyama Y, Fayad ZA, et al. Effect of lipid-lowering therapy with atorvastatin on atherosclerotic aortic plaques detected by noninvasive magnetic resonance imaging. J Am Coll Cardiol 2005;45:733-42. [Crossref] [PubMed]
- de Sauvage Nolting PR, de Groot E, Zwinderman AH, et al. Regression of carotid and femoral artery intima-media thickness in familial hypercholesterolemia: treatment with simvastatin. Arch Intern Med 2003;163:1837-41. [Crossref] [PubMed]
- Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 2003;290:2292-300. [Crossref] [PubMed]
- Räber L, Taniwaki M, Zaugg S, et al. Effect of high-intensity statin therapy on atherosclerosis in non-infarct-related coronary arteries (IBIS-4): a serial intravascular ultrasonography study. Eur Heart J 2015;36:490-500. [Crossref] [PubMed]
- Amarenco P, Labreuche J, Lavallée P, et al. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke 2004;35:2902-9. [Crossref] [PubMed]
- Schartl M, Bocksch W, Koschyk DH, et al. Use of intravascular ultrasound to compare effects of different strategies of lipid-lowering therapy on plaque volume and composition in patients with coronary artery disease. Circulation 2001;104:387-92. [Crossref] [PubMed]
- Kawasaki M, Sano K, Okubo M, et al. Volumetric quantitative analysis of tissue characteristics of coronary plaques after statin therapy using three-dimensional integrated backscatter intravascular ultrasound. J Am Coll Cardiol 2005;45:1946-53. [Crossref] [PubMed]
- Toutouzas K, Vaina S, Tsiamis E, et al. Detection of increased temperature of the culprit lesion after recent myocardial infarction: the favorable effect of statins. Am Heart J 2004;148:783-8. [Crossref] [PubMed]
- Furman C, Copin C, Kandoussi M, et al. Rosuvastatin reduces MMP-7 secretion by human monocyte-derived macrophages: potential relevance to atherosclerotic plaque stability. Atherosclerosis 2004;174:93-8. [Crossref] [PubMed]
- Landmesser U, Bahlmann F, Mueller M, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 2005;111:2356-63. [Crossref] [PubMed]
- Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827-32. [Crossref] [PubMed]
- Tenenbaum A, Shemesh J, Koren-Morag N, et al. Long-term changes in serum cholesterol level does not influence the progression of coronary calcification. Int J Cardiol 2011;150:130-4. [Crossref] [PubMed]
- Maniscalco BS, Taylor KA. Calcification in coronary artery disease can be reversed by EDTA-tetracycline long-term chemotherapy. Pathophysiology 2004;11:95-101. [Crossref] [PubMed]
- Budoff MJ, Raggi P. Coronary artery disease progression assessed by electron-beam computed tomography. Am J Cardiol 2001;88:46E-50E. [Crossref] [PubMed]
- Goh VK, Lau CP, Mohlenkamp S, et al. Outcome of coronary plaque burden: a 10-year follow-up of aggressive medical management. Cardiovasc Ultrasound 2010;8:5. [Crossref] [PubMed]
- Terry JG, Carr JJ, Kouba EO, et al. Effect of simvastatin (80 mg) on coronary and abdominal aortic arterial calcium (from the coronary artery calcification treatment with zocor [CATZ] study). Am J Cardiol 2007;99:1714-7. [Crossref] [PubMed]
- Schmermund A, Achenbach S, Budde T, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation 2006;113:427-37. [Crossref] [PubMed]
- Raggi P, Davidson M, Callister TQ, et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES). Circulation 2005;112:563-71. [Crossref] [PubMed]
- Arad Y, Spadaro LA, Roth M, et al. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol 2005;46:166-72. [Crossref] [PubMed]
- Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49-57. [Crossref] [PubMed]
- Park HB, Heo R. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging 2015;8:1-10. [Crossref] [PubMed]
- Erbel R, Lehmann N, Churzidse S, et al. Progression of coronary artery calcification seems to be inevitable, but predictable - results of the Heinz Nixdorf Recall (HNR) study. Eur Heart J 2014;35:2960-71. [Crossref] [PubMed]
- Puato M, Faggin E, Rattazzi M, et al. Atorvastatin reduces macrophage accumulation in atherosclerotic plaques: a comparison of a nonstatin-based regimen in patients undergoing carotid endarterectomy. Stroke 2010;41:1163-8. [Crossref] [PubMed]
- Hattori K, Ozaki Y, Ismail TF, et al. Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter-IVUS. JACC Cardiovasc Imaging 2012;5:169-77. [Crossref] [PubMed]
- Shaw LJ, Narula J, Chandrashekhar Y. The never-ending story on coronary calcium: is it predictive, punitive, or protective? J Am Coll Cardiol 2015;65:1283-5. [Crossref] [PubMed]
- Weijs B, Blaauw Y, Rennenberg RJ, et al. Patients using vitamin K antagonists show increased levels of coronary calcification: an observational study in low-risk atrial fibrillation patients. Eur Heart J 2011;32:2555-62. [Crossref] [PubMed]
- Hruby A, O'Donnell CJ, Jacques PF, et al. Magnesium intake is inversely associated with coronary artery calcification: the Framingham Heart Study. JACC Cardiovasc Imaging 2014;7:59-69. [Crossref] [PubMed]


