Long term responders to palliative chemotherapy for advanced biliary tract cancer
Introduction
Biliary tract cancer (BTC) encompasses tumors of the gallbladder, intrahepatic and extrahepatic bile ducts. These are rare cancers in most of the world, but significant regional variation in incidence exists with East Asia and Latin America reporting rates higher than international averages (1). Gallbladder cancer is falling in incidence worldwide, but rates of intrahepatic cholangiocarcinoma appear to be rising (2). Curative treatment relies upon surgical excision, but even those with localized disease have survival rates of 30% at 5 years from registry data (3). In patients with metastatic or locally advanced BTC, there has been limited progress in treatment. Early studies of chemotherapy demonstrated responses to 5-fluorouracil (5-FU) and gemcitabine, with response rates ranging from 10–30% (4,5). The combination of gemcitabine and capecitabine has also been studied, with median overall survival (OS) of 14 months in a phase II study (6). In recent years, the standard chemotherapy regimen for advanced BTC has consisted of cisplatin and gemcitabine, based on the results of the randomized phase III ABC-02 study which demonstrated superiority of this regimen with median OS of 11.7 months, compared with median OS of 8.1 months for gemcitabine alone. Progression-free survival (PFS) also favored the doublet over single agent (8 vs. 5 months) (7). There is no established second-line chemotherapy regimen in patients with progressive disease on palliative chemotherapy (PC), with studies to date reporting disappointing response rates less than 10% and median PFS times of approximately 3 months (8,9). In the ABC-02 study, patients were treated to 8 cycles of chemotherapy and then discontinued regardless of ongoing response. In clinical practice, patients responding to chemotherapy without significant toxicity often continue chemotherapy for additional cycles. There is a need to consider carefully the balance of quality of life and treatment toxicity with cancer control in the setting of palliative treatment. In breast cancer and lung cancer, there is evidence that continued palliative systemic therapy may impact on patient survival, but the same data are lacking in BTC (10,11).
The aim of this study was to review the clinical features and outcomes of patients who had longer than expected treatment with PC for BTC, and to compare these features and outcomes with other patients at our institution who were treated with PC. It was hypothesized that patients treated with 9 or more cycles would have better survival than those treated with fewer cycles, and that some clinical or demographic features may be identifiable to predict which patients derive greater benefit.
Methods
This retrospective study included patients treated with 2 or more cycles of PC for BTC at Princess Margaret Cancer Centre between 1987 and 2015. These data were collected as part of the institutional database of BTC patients, including all patients treated at Princess Margaret Cancer Centre. Included patients were required to have a pathological or cytological diagnosis of gallbladder carcinoma, intrahepatic or extrahepatic cholangiocarcinoma. Patients with mixed hepatocellular/cholangiocarcinoma were excluded, as were peri-ampullary cancers and those who discontinued chemotherapy after only one cycle. Institutional Review Board approval was obtained for the study (07-0376-CE).
The following baseline data were collected from the records of included patients: age, sex, date of diagnosis, primary tumor site (intrahepatic, hilar, distal bile duct, gallbladder), Eastern Cooperative Oncology Group (ECOG) performance status at diagnosis, symptoms at diagnosis (pain, jaundice, weight loss, nausea/vomiting), tumor stage, tumor grade and differentiation, history of definitive surgical resection, previous adjuvant chemotherapy, history of biliary stenting, date of recurrence, and pattern of recurrence. Data regarding PC were also collected: start and end date of PC, chemotherapy regimen, number of cycles, toxicities (neutropenia, thrombocytopenia, skin toxicity, nausea/vomiting, diarrhea, peripheral neuropathy, nephropathy, anemia), response to chemotherapy, reason for discontinuation, date of progression, and details of second-line chemotherapy (if used). Toxicities were not graded as per CTCAE, but recorded if considered clinically significant by the following measures: resulted in delay, dose reduction or discontinuation of chemotherapy, or required specific intervention (for example, transfusion for anemia).
Pathological and clinical staging was based on the seventh edition, American Joint Committee on Cancer Tumor Node Metastasis staging system, even for the earliest period in which tumors were retrospectively staged (12). Tumor regression was defined as any tumor shrinkage from baseline prior to commencement of chemotherapy as per radiology reporting. As most of these patients were treated outside of a clinical trial, formal response evaluation was not performed, but standard reporting followed RECIST practice. Long term responders (LTR) were defined as those who had received 9 or more cycles of first-line PC, and those who had 2–8 cycles were included as a control group. No matching of cases and controls was performed as this was a retrospective study. Regarding chemotherapy regimens, the fluoropyrimidines 5-FU and capecitabine were used and these were grouped together for this analysis. Patients treated with selumetinib as an experimental agent with cisplatin and gemcitabine as part of a clinical trial were also included.
Patient demographics and clinical characteristics were summarized using descriptive methods, and differences between treatment groups at baseline were evaluated using Chi-square, Fisher’s exact test and t-tests as appropriate. The two primary event outcomes were PFS and OS. PFS in this study was defined as time from start of PC to disease progression, as per treating physician or death from any cause. OS was defined as the time from start of PC to death from any cause. T Survival probabilities were estimated using the Kaplan-Meier method. Survival differences between groups were examined using log rank tests. Cox proportional hazard models were developed using relevant clinicopathological variables to determine the association of each with OS. Variables with a P<0.05 in univariable analysis were included in a multivariable model. Results were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). All tests were two-sided. A P value of <0.05 was considered statistically significant. Analyses were performed using SAS (Statistical Analysis System, version 9.4) and R 3.0.0.
Results
Patient characteristics
Between 1987 and 2015, a total of 1558 patients were identified as having been treated for BTC (all stages) at Princess Margaret Cancer Centre. Of these, 382 had 2 or more cycles of PC for advanced disease and were included in this study. Patients were divided into two groups for analysis: 123 patients who had 9 or more cycles of first-line chemotherapy (LTR), and 259 who had 2–8 cycles (controls). There were no significant differences in age, sex, period of diagnosis, presence of biliary stent, tumor grade or ECOG performance status at diagnosis (Table 1). There was a difference in distribution of patients across biliary cancer subtypes, with more patients in the LTR group with intrahepatic and hilar cholangiocarcinoma, and more distal bile duct and gallbladder cancers in the control group (P=0.024). More patients in the control group had previous definitive surgery before recurrence (39% vs. 26%, P=0.016), but rates of adjuvant chemotherapy and median time to recurrence after surgery in these patients were similar.
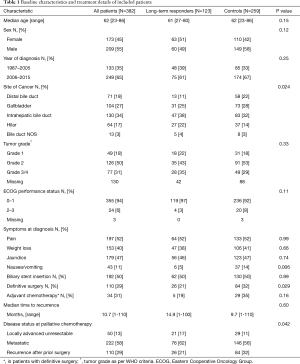
Full table
Details of treatment for advanced disease
Patients in the LTR group were more frequently treated with combination chemotherapy regimens than those in the control group (93% vs. 82%, respectively, P=0.003). In both groups, most patients were treated with either gemcitabine and cisplatin or gemcitabine and a fluoropyrimidine (capecitabine or 5-FU, Table 2). There was a difference in the distribution of chemotherapy regimens between groups, with more patients in the LTR group receiving fluoropyrimidine-gemcitabine combinations, and more patients in the control group treated with platinum-gemcitabine combinations (P=0.007). In the LTR group, the median number of chemotherapy cycles was 12 (range 9–47), compared with 4 in the control group (range 2–8, P<0.001). The reasons for stopping chemotherapy were not different between LTR and control groups: disease progression (radiological and/or clinical) was the commonest reason (56% and 58%), followed by planned discontinuation and toxicity. In both groups, 6% stopped due to other reasons. These included episodes of sepsis, surgical procedures and upper gastrointestinal hemorrhage. More frequent hematologic toxicity was observed in the LTR group than in controls, in the form of neutropenia (30% vs. 14%, P<0.001) and thrombocytopenia (26% vs. 17%, P=0.056, Table 2). There was also more frequent skin toxicity in this group (32% vs. 12%, P<0.001), which may relate to the increased proportion of patients treated with a fluoropyrimidine in the LTR group or from overall treatment exposure. In analysis of toxicity by chemotherapy regimen, there was a significantly higher rate of skin toxicity (34%) in patients treated with gemcitabine/fluoropyrimidine chemotherapy (34%) than other regimens (P<0.001, Table S1). In contrast, neuropathy and nephropathy were more common with gemcitabine/cisplatin chemotherapy, as expected (P=0.002 for both). In both groups, a small number of patients treated with combination regimens were changed to single agent chemotherapy—6 (5%) in the LTR group (at a median of 8 cycles) and 7 (3%) in the control group (at a median of 5 cycles). In the LTR group, 24 patients (20%) stopped first-line chemotherapy for 3 months or more, and restarted the same regimen on progression and achieved disease control again; no patients in the control group had such a break. More patients in the LTR group went on to have second-line chemotherapy regimens (49% vs. 33%, P=0.002), although patients in both groups had a median of 3 cycles of second-line chemotherapy. A group of patients in both LTR and control cohorts (12% and 7%, respectively, P=0.08) were treated with stereotactic radiotherapy to their primary tumors, or high-dose fractionated radiotherapy.
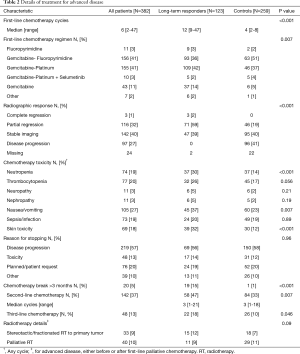
Full table
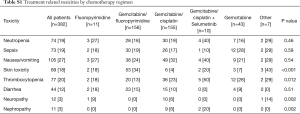
Full table
PFS, radiographic response and OS
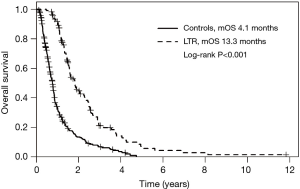
At a median follow up time of 10.7 months (range, 1.3–142 months), the PFS in the entire study cohort was 6.3 months (95% CI, 5.5–7.2). PFS was significantly longer in LTR patients at 13.3 months (95% CI, 11.4–15.4) than in the control group at 4.1 months (95% CI, 3.7–4.6; P<0.001, Figure 1). There was significantly higher rate of radiological regression from baseline prior to commencement of PC in the LTR group than in controls (62% vs. 19%, P<0.001), a similar rate of stable imaging as best response (39% vs. 40%) and a lower rate of disease progression (0 vs. 41%) as best response. The absence of patients with progression as best response was expected given the selection of LTR patients as those treated with 6 months of chemotherapy—patients with progression at first response assessment would have stopped first-line chemotherapy at this early time point.
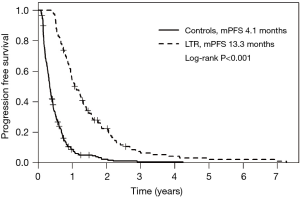
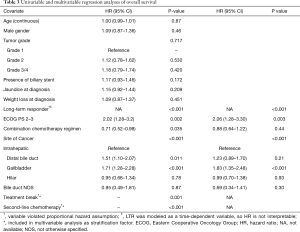
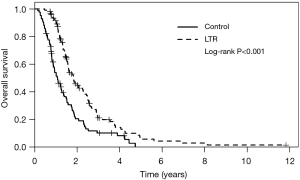
Median OS in the entire study cohort was 12.9 months (95% CI, 11.3–14.2) and was longer in the LTR group at 22.1 months (range, 6–142 months) compared with 9.2 months (range, 1–57 months) in the control group (95% CI, 8.0–10.0; P<0.001, Figure 2). In addition, a small number of patients had true long term survival following PC, with 9 patients in the LTR group alive at 4 years, and 2 alive at 8 years. This analysis did not conform to the proportional hazards model, as long term response was a time-dependent variable. As such, HRs for LTR survival at 1 year and 2 years were 0.29 (95% CI, 0.21–0.40) and 0.69 (95% CI, 0.47–1.01), respectively. Other variables associated with better OS in univariable models included intrahepatic site of primary tumor (with gallbladder cancer associated with the worst outcomes), good performance status at diagnosis, treatment with second-line chemotherapy, and treatment break (defined as >3 months with no treatment, followed by re-initiation of the same regimen); these results are summarized in Table 3. Survival in patients with locally advanced disease was not different to those with distant metastases or recurrence after surgery (P=0.37), and no significant differences were seen between chemotherapy regimen (P=0.16). Variables found to be significant at a univariable level were included in a multivariable regression model, and long term response remained significantly associated with better OS at this level (P<0.001). Receipt of second line chemotherapy was also independently associated with longer OS on multivariable analysis (P<0.001). Poor performance status (ECOG 2–3) was independently associated with shorter OS (HR 2.06, P=0.003), and the association between site of cancer and survival was maintained in the multivariable model (P<0.001). Patients in the control group, who received second-line chemotherapy, still had shorter OS than LTR patients (P<0.001, Figure S1), illustrating that the effect of continued first-line chemotherapy was greater than two separate treatment lines.
Full table
Discussion
The best results from clinical trials of chemotherapy in advanced BTC suggest a median survival of less than 1 year, even with the use of multi-agent regimens (7). In this large cohort of patients treated with chemotherapy for advanced BTC, 30% of patients were treated with 9 or more cycles of first-line therapy. This was a group of patients who were symptomatic or had significant disease progression, some with deteriorating performance status at the start of PC. In spite of this, a considerable proportion had significantly longer than expected progression-free and OS with continued chemotherapy, at the cost of some increased rates of some toxicities. Analysis of clinical characteristics did not reveal any prominent features predictive of benefit from chemotherapy. If chemotherapy had been stopped at 8 cycles (as per trial data), it is likely that some of this benefit would have been missed. In addition, a small number of patients in the LTR group had continued benefit from chemotherapy, with 9 alive at 4 years, and 2 alive at 8 years. In this study population, other factors associated with differences in survival included site of primary tumor and performance status.
Recently, a sub-group of patients with long-term survival have been reported from the ABC-02 study (13). Of 410 total patients, 45 (approximately 11%) had continued study follow-up for more than 24 months, with a median survival of 31.4 months. Factors associated with longer survival in that population were chemotherapy regimen (cisplatin/gemcitabine doublet compared with gemcitabine alone), locally advanced disease (compared with metastatic disease), and better ECOG performance status. They did not report data on number of treatment cycles, use of treatment breaks or second-line chemotherapy. In contrast to our population they did not note a significant difference by primary tumor site, although they did not sub-categorize cholangiocarcinoma into intra- or extrahepatic. Our cohort of long-term survivors was a larger proportion of total patients with BTC receiving chemotherapy (30%), and in contrast to the ABC-02 cohort, these patients were treated to disease progression. In the treatment of other cancers, the use of chemotherapy to disease progression has been associated with longer survival. In patients with metastatic breast cancer, a systematic review of studies reported that continuing chemotherapy to disease progression was associated with a modest but significant effect on OS (HR 0.91, 95% CI, 0.84–0.99) (10). Studies of “maintenance” chemotherapy to disease progression in lung cancer have also shown improvements in survival with this strategy (HR 0.78, P=0.0195 for pemetrexed) (14). In patients with metastatic colon cancer, the use of maintenance 5-FU chemotherapy after combination 5-FU, leucovorin and oxaliplatin (FOLFOX) was associated with longer disease control than a chemotherapy-free interval (13.1 vs. 9.2 months, P=0.46), but no significant difference in OS was noted (15).
Although tumors of the biliary tract are treated similarly, there is now a significant body of evidence demonstrating significant differences in the molecular pathogenesis and clinical outcomes of cholangiocarcinomas of the intrahepatic bile ducts, extrahepatic bile ducts and cancer of the gallbladder (16,17). There have been a number of reports of distinct molecular drivers in tumors of different sites, with IDH1/2 (isocitrate dehydrogenase 1/2) mutations and FGFR (fibroblast growth factor receptor) fusion events in intrahepatic tumors, PRKACA (Protein Kinase CAMP-Activated Catalytic Subunit Alpha) fusions and ARID1B (AT-Rich Interaction Domain 1B) mutations in extrahepatic cholangiocarcinoma, and EGFR (epidermal growth factor receptor) and ERBB3 (Erb-B2 Receptor Tyrosine Kinase 3) mutations seen in gallbladder cancers. In our study, patients with gallbladder cancer had worse outcomes than patients with intrahepatic cholangiocarcinoma (HR 1.81), although patients with other BTC subtypes had similar survival times. This finding is consistent with previous reports across biliary cancer subtypes, with a prior pooled analysis of clinical trials of chemotherapy in advanced BTC reporting better response rates in gallbladder cancer but shorter OS (18).
Our study has some limitations. The retrospective nature of this analysis introduces bias, and limits the applicability of its results. In addition, there is heterogeneity in the chemotherapy regimens used in these patients, but most patients received doublet chemotherapy with gemcitabine and either a fluoropyrimidine or platinum agent. Relatively few patients in the LTR group received single agent chemotherapy, but documentation of reason for choice of chemotherapy agent was not always possible to collect. Toxicity rates were higher in the LTR group, as expected with longer chemotherapy exposure. No quality-of-life data are available for this group, but almost half went on to receive second-line chemotherapy, demonstrating that their performance status and willingness to have treatment was intact. Some patients in this study had next-generation sequencing of their tumors performed, but the numbers were small in each group and could not be used to draw any comparisons. There has not yet been a report of any molecular characteristic that can predict response or long-term disease control from chemotherapy for BTC, and further biomarker discovery in this population is warranted.
In summary, we report the outcomes of patients treated with PC for advanced BTC at our institution, and describe a significant size group that appear to derive benefit from continuing chemotherapy for more than 8 cycles. No major clinical features were noted to distinguish this group from other patients treated with similar chemotherapy regimens other than the lack of disease progression at 8 cycles. Clinicians should be aware of potential for longer-term benefit from chemotherapy in a subset of patients with BTC, and consider the rationale for continued treatment in the absence of cumulative toxicity. Prospective studies with molecular correlates are necessary to further explore this finding and develop better treatment strategies for those who experience limited benefit from current therapies.
Acknowledgements
Mark K Doherty was supported by the Thompson Biliary Fund administered through the Princess Margaret Cancer Foundation. The funding source had no role in the conduct of the study or the preparation of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional Review Board approval was obtained for the study (07-0376-CE).
References
- Randi G, Malvezzi M, Levi F, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol 2009;20:146-59. [Crossref] [PubMed]
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353-7. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. National Cancer Institute, Bethesda, 2016. Available online: http://seer.cancer.gov/csr/1975_2013/
- Falkson G, MacIntyre JM, Moertel CG. Eastern Cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer 1984;54:965-9. [Crossref] [PubMed]
- Kornek GV, Schuell B, Laengle F, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol 2004;15:478-83. [Crossref] [PubMed]
- Knox JJ, Hedley D, Oza A, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 2005;23:2332-8. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Walter T, Horgan AM, McNamara M, et al. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer 2013;49:329-35. [Crossref] [PubMed]
- Lamarca A, Hubner RA, David Ryder W, et al. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 2014;25:2328-38. [Crossref] [PubMed]
- Gennari A, Stockler M, Puntoni M, et al. Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol 2011;29:2144-9. [Crossref] [PubMed]
- Lima JP, dos Santos LV, Sasse EC, et al. Optimal duration of first-line chemotherapy for advanced non-small cell lung cancer: a systematic review with meta-analysis. Eur J Cancer 2009;45:601-7. [Crossref] [PubMed]
- American Joint Committeeon Cancer. AJCC Cancer Staging Manual. 7th ed. Springer, New York/London, 2011.
- Bridgewater J, Lopes A, Palmer D, et al. Quality of life, long-term survivors and long-term outcome from the ABC-02 study. Br J Cancer 2016;114:965-71. [Crossref] [PubMed]
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:2895-902. [Crossref] [PubMed]
- Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol 2009;27:5727-33. [Crossref] [PubMed]
- Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003-10. [Crossref] [PubMed]
- Churi CR, Shroff R, Wang Y, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One 2014;9:e115383. [Crossref] [PubMed]
- Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007;96:896-902. [Crossref] [PubMed]


