Robotic enucleation of benign pancreatic tumors
Introduction
Surgical resection is the only potentially curative treatment for benign and borderline neoplasms of the pancreas. Robot-assisted enucleation provides the dual benefits of a minimally-invasive technique and pancreatic parenchymal conservation to selected patients with functional neuroendocrine tumors (pNETs) and serous cystadenomas. This review describes the technique of robot-assisted enucleation with an up to date description of indications, patient selection, pre-operative evaluation, and post-operative outcomes.
Patient selection
The 2017 NCCN guidelines (1) for functional pancreatic neuroendocrine tumors (F-pNETs) recommend enucleation for superficial insulinomas, gastrinomas, and VIPomas and peripancreatic lymphadenectomy reserved for gastrin and VIP-secreting lesions (2). Enucleation is usually reserved for solitary pancreatic lesions <2 cm in diameter given the link between tumor size and risk for malignancy and metastasis, but has been reported for benign tumors greater than 4 cm, suggesting that tumor type and distance to the main pancreatic duct are more important than tumor size alone (3,4). Insulinoma is the most common functional neuroendocrine tumor of the pancreas and are ideal candidates for enucleation when <2 cm given 80% probability that such lesions are benign (5,6).
Whereas most insulinomas are benign, other functional pancreatic neuroendocrine tumors such as gastrinomas, VIPomas, glucagonomas, and somatostatinomas have a higher incidence of malignancy and are more controversial targets for enucleation. Preoperative staging is necessary to rule out local invasion or metastasis. Contrast-enhanced CT and MRI detect liver metastasis with 94% sensitivity and demonstrate tumor number and location as well as distance to the main pancreatic duct. Sensitivity ranges between 55–78% for smaller lesions like insulinomas and gastrinomas (4,7,8). Although endoscopic ultrasound (EUS) detects small lesions with a sensitivity of approximately 90% and permits cytological confirmation, the radial detector provides less useful anatomic localization for operative planning (9). Somatostatin receptor-based PET scan can be used to detect metastatic insulinoma and guide medical treatment with somatostatin analogues (2). Specialized studies including arterial calcium stimulation and hepatic venous sampling are now used only sporadically to localize lesions that cannot be identified on imaging studies (10).
Enucleation of nonfunctional pancreatic neuroendocrine tumors (NF-pNETs) should be approached with greater caution (11). Triponez et al. reported a correlation between the size of NF-pNETs and the risk of distant metastases, rising from 4% for lesions ≤1 cm, 10% between 1.1–2 cm, and 43% when the tumor was >3 cm (6). Current guidelines do not recommend enucleation for NF-pNETs >1–2 cm or for lesions <1 cm with significant growth in the prior 3–6 months (4).
Currently, pNETs share the same TNM/AJCC staging system with pancreatic exocrine tumors albeit with significantly better survival outcomes. The World Health Organization (WHO) recommends histological grading of gastroenteropancreatic neuroendocrine tumors according to mitotic rate and Ki-67 index (2). Current evidence reserves enucleation for lesions meeting specific characteristics (Table 1).
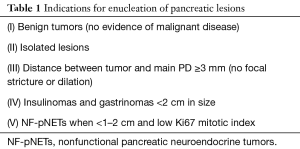
Full table
Evaluation for hormonal activity
Approximately 30–80% of patients with MEN1 syndrome harbor functional pNETs (4), which may be multifocal. Distinguishing sporadic pNET from MEN1 is critical since MEN1 patients may harbor multifocal disease for which medical management is initially indicated (2). Clinical suspicion for MEN1 requires assessment for multi-gland parathyroid hyperplasia and pituitary tumors (2).
The preoperative biochemical evaluation should be guided by the suspected clinical syndrome. Insulin and gastrin (Zollinger-Ellison syndrome, ZES) are the most common hormones produced by F-pNETs. Insulinomas typically present with symptoms of neuroglycopenia associated with high insulin (>3 mcIU/mL) levels, elevated C-peptide (>0.6 ng/mL) and proinsulin concentrations (>5 pmol/L) (6) during fasting hypoglycemia (<55 mg/dL). Insulinomas are potentially dangerous tumors, and hypoglycemia must be addressed with diet or diazoxide so that localization may be safely obtained (12). Gastrinomas may present with recurrent peptic ulcers, diarrhea, and steatorrhea and manifest as elevated fasting serum gastrin concentration (>10 times elevated) with abnormal basal gastric acid secretion (pH <2) (6). Symptoms may be controlled preoperatively with high-dose proton pump inhibitors (13) and octreotide as required (14).
Technique of robot-assisted enucleation
Suggested equipment
- A 5-mm optical separator for peritoneal entry;
- A 12-mm Versaport trocar for the robotic camera;
- A 5-mm Maryland Ligasure energy device;
- A 5-mm suction irrigator;
- Intraoperative ultrasound;
- Da Vinci Robotic Surgery System with fenestrated bipolar; Prograsp; cautery hook; and possibly large needle drivers (robotic instruments).
Patient positioning
The patient is placed on the operating table in a well-padded split leg supine position with a gel-padded foot board. The arm corresponding to the side of the lesion is tucked, and the other arm remains exposed for anesthesia access.
Operative technique
Step 1: port placement
We enter the peritoneal cavity in the left midclavicular line approximately three fingerbreadths below the costal margin using a 5-mm optical separator. Six-Seven ports are required: a 5 mm port in the right anterior axillary line to secure the liver retractor; a 12 mm port in the right lower quadrant for ultrasound access and needle passage; a 12 mm camera port located in proximity to the tumor, and three 8 mm robotic ports across the upper abdomen, with the two robot arms on the side of the tumor (Figures 1,2).
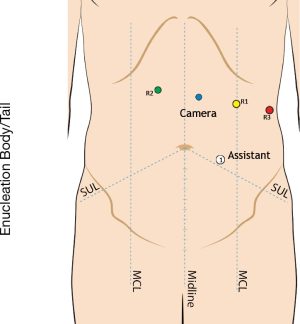
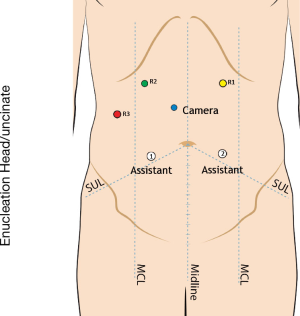
Step 2: exposure
For lesions of the pancreatic head and uncinate process, the lesser sac is divided and a generous Kocher maneuver is performed to expose the pancreatic head and root of the mesentery. The right gastroepiploic vascular pedicle may be divided to expose the medial border of the pancreatic head and uncinate process. Exposure of the superior mesenteric vein may be required to protect it during enucleation of uncinate process lesions and those over the pancreatic neck.
For tumors of the pancreatic body/tail, the greater omentum is divided as far to the left as required to gain adequate exposure, which may require dividing the short gastric vessels as well as splenic flexure omentum along the inferior border of the pancreas.
Step 3: localizing the lesion
With the anterior surface of the pancreas in view and the stomach retracted, we dock the robot prior to intraoperative ultrasound. Minimally-invasive enucleation is an imaging-dependent procedure that requires recognizable anatomic landmarks for successful completion. Critical information includes tumor proximity to the pancreatic duct as well as localization relative to major structures such as the gastroduodenal artery or bile duct, as well as the portal vein behind the pancreatic neck. Intraoperative palpation is not feasible. Localization often mandates intraoperative ultrasound aided by duplex studies of intratumoral blood flow and frozen section confirmation. We utilize the console’s dual visual/ultrasound image platform to localize the lesion and mark the boundaries of enucleation and to confirm proximity to the main pancreatic duct and adjacent major vascular structures (Figure 3). The patient cart’s bulk effectively precludes intraoperative palpation through a hand access port.

Step 4: enucleating the lesion
The pancreatic parenchyma around the lesion is marked with cautery scissors at the desired margin distance, and a silk suture is used for traction and exposure during dissection. The pancreas is divided sequentially using cautery scissors, with 5-0 Prolene and suction used liberally to maintain visualization of the pseudocapsule which marks the minimal acceptable pathological margin (Figure 4).

Step 5: continuity of the pancreatic duct
In the absence of visual evidence that the pancreatic duct has been injured, intraoperative ultrasound is deployed to inspect the deep margin of resection. When in doubt, secretin can be administered while the cavity is observed for signs of a leak. We routinely use a 19 French surgical drain in expectation of a low-output pancreatic fistula.
Post-operative management
An oral diet and pain regimen may be initiated rapidly after minimally-invasive enucleation with expectation of early discharge. Medications, such as diazoxide, used to manage hormone-producing tumors must be adjusted after resection. Surgical drain management is institution-dependent and was not the subject of recent level 1 evidence gathered after pancreaticoduodenectomy or distal pancreatectomy (17).
Postoperative outcomes
The European Association for Endoscopic Surgery Clinical Consensus (13) concluded that minimally-invasive enucleation offered reduced operative time, blood loss and postoperative pain compared to an open approach. Jin et al. (18) compared robotic (n=16) to standard open enucleation (n=19) and found shorter operative time (mean 100 min; range, 90–120 min for robotic vs. mean 140 min; range, 113–193 min for open; P=0.009) without conversions. Blood loss was reduced, but the difference was not clinically significant (median 30 mL robotic vs. 100 mL open; P=0.001). Time to drain removal and discharge were not significantly reduced. Shi et al. reported robotic pancreatic enucleation for isolated lesions at least 1–2 mm away from the main pancreatic duct as measured by MRI (3). Mean tumor size was 23 mm, located in the following regions: neck, body, and tail (58%) vs. head or uncinate process (42%) (3). Outcomes after robotic enucleation (n=26) demonstrated reduced blood loss and operative time compared to open (n=17) but smaller mean tumor size. No differences in morbidity, post-operative stay, or pancreatic fistula rates were observed. No postoperative diabetes or pancreatic exocrine insufficiency developed in the robotic group. Similar data has been reported after enucleation of ≤ two lesions of 1 cm-diameter or less, in the body or tail (6).
Pancreatic fistula remains the principal concern after enucleation. Univariate analysis of fistula risk by Jin et al. (18) reported two important correlations: distance between tumor and the main pancreatic duct as well as operative time. Tian et al. conducted a retrospective review of 60 patients who underwent robotic enucleation for benign pNETs <2 cm diameter with a distance >2 mm from the main pancreatic duct (8). Propensity score matching was used to compare 61 robotic enucleations with 187 open procedures and demonstrated no significant difference in pancreatic fistula rates (17% open vs. 10% robotic) based on operative approach.
Five patients have undergone robot-assisted enucleation at Beth Israel Deaconess Medical Center between January 2014 and January 2017. Mean age was 56 years (range, 49–66 years) with median tumor diameter of 1.3 cm (0.9–1.7 cm) located in the pancreatic head [2] and tail [3]. Surgical indications included insulinoma [2] and NF-pNETs [3]. Median operative time was 204 min (range, 137–347 min) with 50 mL median estimated blood loss and no conversions or transfusions. One patient developed a post-operative pancreatic fistula. Median time to oral diet was 2 days (1,2). All patients were discharged with a drain. There were no readmissions or deaths at 90 days.
Tips, tricks and pitfalls
- Hormonally active neuroendocrine tumors should be evaluated prior to surgery and medicated appropriately to optimize perioperative recovery.
- Preoperative cross-sectional imaging and endoscopic intra-operative ultrasound (IOUS) should be used to establish anatomic boundaries for enucleation and estimate proximity to the main pancreatic duct.
- Intraoperative ultrasound should be the surgeon’s GPS system during enucleation to minimize the risk of margin contamination or pancreatic duct injury.
- Enucleation may require a row of parenchymal sutures to control bleeding during dissection. Bleeding obscures the deep surface of the operative field and may endanger critical structures such as the pancreatic duct, portal or splenic veins, and gastroduodenal or splenic arteries.
- The surgical margin should be carefully scrutinized to be certain that tumor is not left behind. This may require frozen section evaluation or specimen ultrasound.
- Post-enucleation ultrasound is mandatory to confirm the integrity of the pancreatic duct. Secretin may be helpful in equivocal cases.
Conclusions
Robotic enucleation is safe and feasible, providing parenchymal conservation in a minimally-invasive setting that reduces operative time and length of stay with equivalent pathological outcomes. Larger studies are needed to confirm these emerging data.
Acknowledgements
Funding: The Alliance of Families Fighting Pancreatic Cancer, the Greg and Cathy Griffith Family Foundation, and the John F. Fortney Charitable Pancreatic Cancer Research Group.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- NCCN. Pancreatic Adenocarcinoma Guideline 2017.
- Zhang T, Xu J, Wang T, et al. Enucleation of pancreatic lesions: indications, outcomes, and risk factors for clinical pancreatic fistula. J Gastrointest Surg 2013;17:2099-104. [Crossref] [PubMed]
- Shi Y, Peng C, Shen B, et al. Pancreatic enucleation using the da Vinci robotic surgical system: a report of 26 cases. Int J Med Robot 2016;12:751-7. [Crossref] [PubMed]
- Mehrabi A, Fischer L, Hafezi M, et al. A systematic review of localization, surgical treatment options, and outcome of insulinoma. Pancreas 2014;43:675-86. [Crossref] [PubMed]
- Lopez CL, Albers MB, Bollmann C, et al. Minimally Invasive Versus Open Pancreatic Surgery in Patients with Multiple Endocrine Neoplasia Type 1. World J Surg 2016;40:1729-36. [Crossref] [PubMed]
- Triponez F, Dosseh D, Goudet P, et al. Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg 2006;243:265-72. [Crossref] [PubMed]
- Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab 2012;97:2990-3011. [Crossref] [PubMed]
- Tian F, Hong XF, Wu WM, et al. Propensity score-matched analysis of robotic versus open surgical enucleation for small pancreatic neuroendocrine tumours. Br J Surg 2016;103:1358-64. [Crossref] [PubMed]
- Clark OH, Benson AB 3rd, Berlin JD, et al. NCCN Clinical Practice Guidelines in Oncology: neuroendocrine tumors. J Natl Compr Canc Netw 2009;7:712-47. [Crossref] [PubMed]
- Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 2008;135:1469-92. [Crossref] [PubMed]
- Sauvanet A, Gaujoux S, Blanc B, et al. Parenchyma-sparing pancreatectomy for presumed noninvasive intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 2014;260:364-71. [Crossref] [PubMed]
- Lu WJ, Xu B, Gao SL, et al. Enucleation of benign or borderline pancreatic head tumors adjacent to the common pancreatic duct. Pancreas 2012;41:336-7. [Crossref] [PubMed]
- Gut P, Waligórska-Stachura J, Czarnywojtek A, et al. Management of the hormonal syndrome of neuroendocrine tumors. Arch Med Sci 2017;13:515-24. [Crossref] [PubMed]
- Chan DL, Ferone D, Albertelli M, et al. Escalated-dose somatostatin analogues for antiproliferative effect in GEPNETS: a systematic review. Endocrine 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Ore AS, Barrows CE, Solis-Velasco M, et al. IOUS during robotic benign enucleation. Available online: http://www.asvide.com/articles/1759
- Ore AS, Barrows CE, Solis-Velasco M, et al. Preservation of tumor pseudocapsule during robotic benign enucleation. Available online: http://www.asvide.com/articles/1760
- Van Buren G 2nd, Bloomston M, Schmidt CR, et al. A Prospective Randomized Multicenter Trial of Distal Pancreatectomy With and Without Routine Intraperitoneal Drainage. Ann Surg 2017;266:421-31. [Crossref] [PubMed]
- Jin JB, Qin K, Li H, et al. Robotic Enucleation for Benign or Borderline Tumours of the Pancreas: A Retrospective Analysis and Comparison from a High-Volume Centre in Asia. World J Surg 2016;40:3009-20. [Crossref] [PubMed]
Cite this article as: Ore AS, Barrows CE, Solis-Velasco M, Shaker J, Moser AJ. Robotic enucleation of benign pancreatic tumors. J Vis Surg 2017;3:151.


