Reasons for conversion during VATS lobectomy: what happens with increased experience
Introduction
Although an increasing number of studies in literature have found that video-assisted thoracic surgery (VATS) lobectomy appears to be superior to conventional open lobectomy for perioperative outcomes (1-3), extreme caution must be exercised during major pulmonary resections through thoracoscopic approach because the procedure is not without risks. Several authors, in fact, have reported conversion rates to open surgery during VATS lobectomy for a variety of intraoperative reasons: in the range below 3% up to 23% (4,5). Calling in the question the issue of the intraoperative complications during lobectomy via VATS incisions, some researchers have carefully reviewed and classified the reasons for the conversion to thoracotomy in order to make the minimally invasive approach for pulmonary lobectomy a safer procedure (6,7).
Methods
At our institution, between 2011 and 2017, 573 patients underwent VATS lobectomy for known or suspected lung cancer. The VATS approach was converted to open thoracotomy in 40 (6.9%) of 573 patients for a variety of reasons. The length of our learning curve (LC), as also suggested by several authors (8,9), consisted of 50 VATS lobectomies. Patients undergoing conversion to open surgery were divided into two groups: those treated during LC (LC group) and those treated after LC (ALC group). In all cases VATS lobectomy was performed using a three-port anterior approach, with individual dissection of bronchovascular structures and lymph node dissection or sampling without ribs spreading and self-expanding instruments applied to open the wound. All patients underwent single lung ventilation with a double-lumen endotracheal tube.
Results
In the LC group the conversion rate was: 18% (9 out of 50). The patients’ characteristics are listed in Table 1. The most frequent reasons for conversion were absent or incomplete fissure (seven patients), followed by benign hilar or mediastinal adenopathy, vessel injury and pleural symphysis. In all cases a planned conversion to open thoracotomy was performed by extending the utility incision to a standard lateral thoracotomy. The only vascular lesion reported was an iatrogenic injury of a bronchial artery during mediastinal lymph node dissection in patient undergoing left lower lobectomy for treatment of adenocarcinoma. Postoperative complication observed was prolonged air leak (>5 days) in one patient. In the ALC group the conversion rate was: 5.9% (31 out of 523). The patients’ characteristics are listed in Table 2. The most frequent reasons for conversion were vessel injury (thirteen patients), followed by benign hilar adenopathy, calcified hilar adenopathy, vascular adventitial fibrosis, malignant hilar adenopathy, incomplete fissure and vascular anomaly. In four cases, due to vascular injury, surgical team decided to perform an emergent conversion to open thoracotomy. As far as vascular conversion, 77% of the vascular injuries were pulmonary artery injuries, 8% were bronchial artery injuries and 15% were pulmonary venous injuries. Pulmonary arterial bleeding was caused by calcified hilar adenopathy and benign or malignant hilar adenopathy that complicated vascular dissection. Other reasons for conversion, due to pulmonary arterial bleeding, were: accidental movement of instrument around the vessel, forced dissection of a dense vascular structure in patient who received induction therapy, mechanical failure of the stapler (Figure 1) and inadvertent thermal injury to adjacent vascular structure using vessel sealing device (Figure 2). Left and right upper lobectomies were the most frequently associated with conversion to thoracotomy for vessel injury (10/13, 77%). As far as pathological N stage, in the ALC group 26% of the patients (8 out of 31) had postoperative histological diagnosis of N1 or N2 disease. Postoperative complications were observed in eight patients, consisting of atrial fibrillation in five patients, prolonged air leak (>5 days) in two patients and acute respiratory distress syndrome (ARDS) in one patient that led to prolonged intensive care unit (ICU) stay and subsequently to death.
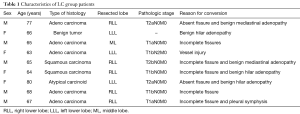
Full table
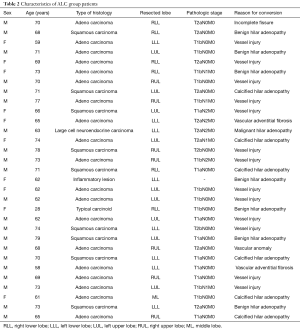
Full table


Discussion
In the literature there is a surprising number of papers written with the objective to study causes of intraoperative conversion to thoracotomy during VATS lobectomy using classification systems too (1,6,12). This single centre retrospective review investigates instead how the reasons for conversion to thoracotomy during VATS lobectomy change with increased experience. At the beginning of our training for major lung resections by VATS, the most frequent cause of conversion to open surgery was absent or incomplete fissure. After an initial learning-curve period, calcified, benign or malignant hilar adenopathy was the leading cause of conversion to thoracotomy due to complicated vascular dissection or vessel injury. This means that, with increased experience, surgeons tend to extend the application of VATS to more advanced disease or more challenging cases. Some authors assert that, regardless of the skills acquired, there is a patient population in which VATS lobectomy is difficult to perform (6); this explains why published intraoperative conversion rates to open thoracotomy range however from 2% to 20% (4,13). In our retrospective review the conversion rate was 18% during the LC but, with increased unit experience, it decreased to 5.9%. In according to other authors, we believe that the reasons for conversion during VATS lobectomy can decline with experience and number of cases for year but don’t disappear altogether (1,6,8). In this study upper lobectomies were the most frequently associated with conversion due to vessel injury (10/13, 77%): our findings would seem to show that vascular lesions can occur more frequently during the upper lobectomies. Among the causes of vessel injury we report a case of stapling failure, during a right lower lobectomy, occurring after the LC: this adverse event suggests that, as the number of VATS lobectomies increases, it is likely that similar intraoperative complications will occur in the future. In our retrospective review, two patients required expeditious conversion to open thoracotomy due to heat injury to adjacent vascular structure using vessel-sealing device: our findings suggest that these instruments require extreme caution during dissection of the hilar structures and the surgical equipment should know the spatial temperature distribution caused by different devices with regard to application time and power setting.
Conclusions
With increased confidence in performing VATS lobectomies, the conversion rate to thoracotomy tend to decrease but, extending minimally invasive surgery to more advanced disease or more complex cases, the risk of intraoperative complications persists. This review proves, moreover, that the reasons for conversion to open surgery change with increased experience and that hilar adenopathy can make thoracoscopic dissection of the hilum technically challenging, increasing the risk of vessel injury. We wish to highlight that thoracoscopic surgeons should always identify the preoperative risk factors to reduce unexpected conversion to thoracotomy and we strongly recommend to follow always rigorous training programs for management strategies in case of severe intraoperative complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Puri V, Patel A, Majumder K, et al. Intraoperative conversion from video-assisted thoracoscopic surgery lobectomy to open thoracotomy: a study of causes and implications. J Thorac Cardiovasc Surg 2015;149:55-61, 62.e1.
- Nwogu CE, D’Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5. [Crossref] [PubMed]
- Nomori H, Horio H, Naruke T, et al. What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? Ann Thorac Surg 2001;72:879-84. [Crossref] [PubMed]
- Sawada S, Komori E, Yamashita M. Evaluation of video-assisted thoracoscopic surgery lobectomy requiring emergency conversion to thoracotomy. Eur J Cardiothorac Surg 2009;36:487-90. [Crossref] [PubMed]
- Park JS, Kim HK, Choi YS, et al. Unplanned conversion to thoracotomy during video-assisted thoracic surgery lobectomy does not compromise the surgical outcome. World J Surg 2011;35:590-5. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg 2012;1:47-50. [PubMed]
- McKenna RJ Jr. Complications and learning curves for video-assisted thoracic surgery lobectomy. Thorac Surg Clin 2008;18:275-80. [Crossref] [PubMed]
- Amore D, Di Natale D, Scaramuzzi R, et al. Vessel injury during VATS right lower lobectomy. Asvide 2018;5:127. Available online: http://www.asvide.com/article/view/23401
- Amore D, Di Natale D, Scaramuzzi R, et al. Bleeding complication during VATS right upper lobectomy. Asvide 2018;5:128. Available online: http://www.asvide.com/article/view/23402
- Watanabe A, Osawa H, Watanabe T, et al. Complications of major lung resections by video-assisted thoracoscopic surgery. Kyobu Geka 2003;56:943-8. [PubMed]
- Gazala S, Hunt I, Valji A, et al. A method of assessing reasons for conversion during video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:962-4. [Crossref] [PubMed]
Cite this article as: Amore D, Di Natale D, Scaramuzzi R, Curcio C. Reasons for conversion during VATS lobectomy: what happens with increased experience. J Vis Surg 2018;4:53.


