Prognostic value of preoperative neutrophil-lymphocyte ratio is superior to platelet-lymphocyte ratio for survival in patients who underwent complete resection of thymic carcinoma
Introduction
Thymic epithelial tumors (TETs), which include thymoma and thymic carcinoma, are most common tumors in the anterior mediastinum (1). Unlike thymoma, thymic carcinoma which accounts for only 0.06% of all thymic neoplasms (2) is aggressive and has a generally poor prognosis, with a 5-year survival rate of approximately 40% (3-5). The complete resection (R0) is the gold standard treatment for operable thymic carcinoma. Radiotherapy and chemotherapy appear to benefit inoperable or incompletely resected patients (6-8).
Over the past decade, the evidence that cancer-related inflammation has a strong influence on outcome in cancer patients has increased and is consistent. A number of inflammation markers have been evaluated to stratify patients for treatment and to predict survival (9). The prognostic value of preoperative systemic inflammation-related neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) has been demonstrated in patients with a variety of cancers (10-18). However, no previous studies have evaluated the association between NLR and PLR with the prognosis of thymic tumors. The purpose of our study was to investigate the prognostic value of preoperative NLR and PLR for patients with thymic carcinoma.
Methods
A total of 90 thymic carcinoma patients underwent complete resection and were treated in the Department of Thoracic Surgery of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China between January 2005 and December 2015. To ensure that the white cell count was representative of a normal baseline, we excluded eleven patients who received preoperative radiotherapy or chemotherapy and those with coexistent hematologic disorders or active infection at the time of surgery. Finally, there were 79 eligible patients with thymic carcinoma included in the study. Complete resection included thymectomy, dissection of mediastinal fat tissue, regional lymph node dissection and resection of adjacent invaded tissues. Regardless of the operative approaches including open surgery and video-assisted thoracoscopic surgery (VATS), all procedures followed the criteria of complete resection. Pathological evaluation of intraoperative frozen sections distinguished thymic carcinoma from other mediastinal masses. Patients who were considered to have a high risk of recurrence based on operative findings were offered postoperative radiotherapy and/or chemotherapy.
This retrospective, observational study evaluated the relationship of a number of cellular markers of systemic inflammation with patient survival. Blood samples of all eligible patients were obtained before surgery. All cases were staged according to the Masaoka system, and histologic classification of thymic carcinoma was based on the World Health Organization (WHO) histologic criteria (19), which were determined by postoperative histopathological analysis and the clinicopathological characteristics of all patients by review of their medical records. The NLR, PLR, absolute neutrophil, lymphocyte, and platelet counts were evaluated to determine whether they were predictive of survival. Overall survival (OS) was defined as the interval between the date of surgery and the date of death from any cause or the last follow-up. Disease-free survival (DFS) was defined as the interval between the date of surgery and the first recurrence or metastasis, or the last follow-up. The study was approved by the Ethics Committee of the Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Because the study was a retrospective analysis of patient data and followed the guidelines of the Declaration of Helsinki, the requirement for patient consent was not required.
Statistical analysis
Values of continuous variables were presented as means ± standard deviation (SD) or medians and range. Patients were divided into equal quartiles according to the 25th, 50th and 75th NLR and PLR percentile. Categorical variables were reported as number and percentages. The Kaplan-Meier method was used to calculate the 1-, 3-, and 5-year OS and 1-, 3-, and 5-year DFS. The variables we entered into the univariate analysis may be associated with prognosis of thymic carcinoma according to previous studies. Variables that were found to be associated with survival in the univariate analysis were further tested in a multivariate model. The association between each continuous variable and stratification by threshold was evaluated using the t-test. The association between each categorical variable and stratification was evaluated using the chi-square test. Kaplan-Meier plots were calculated to estimate survival stratified by a significant indicator; differences were tested by the log-rank test. All tests were two-sided, and P values <0.05 were considered significant. All collected data were analyzed by SPSS 19.0 (IBM Corp. Armonk, NY, USA).
Results
Patient characteristics
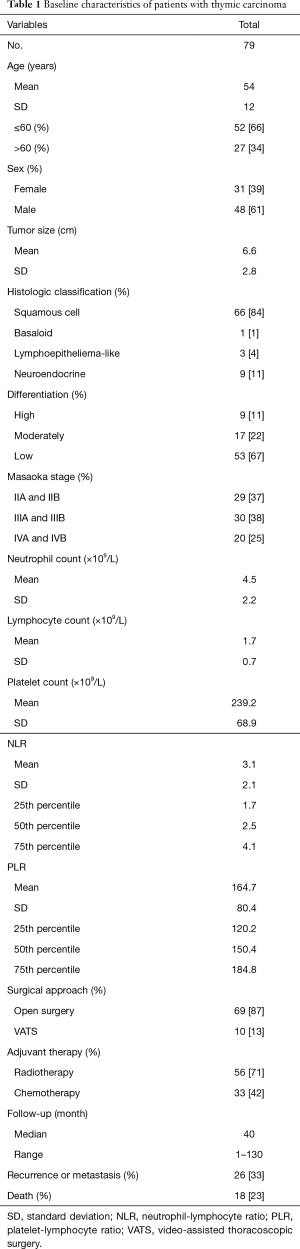
Baseline characteristics of the 79 thymic carcinoma patients are shown in Table 1. There were no perioperative deaths or severe morbidities that were related to the surgical procedures. Median follow-up was 40 months (range, 1–130 months); 18 deaths (23%) and 26 recurrences or metastasis (33%) occurred during that time. The mean age was 54±12 years, 52 (66%) were ≤60 years of age and 27 (34%) were >60 years of age, 31 (39%) were women and 48 (61%) were men. The mean tumor size was 6.6±2.8 cm. Of the four histologic classifications, squamous cell carcinoma was the most common (n=66, 84%), followed by neuroendocrine (n=9, 11%), lymphoepithelioma-like (n=3, 4%), and basaloid (n=1, 1%). Nine patients (11%) had highly differentiated tumors, 17 (22%) had moderately differentiated tumors, and 53 (67%) had low differentiated tumors. Twenty-nine patients (37%) were as Masaoka stages IIA and IIB, 30 (38%) were stages IIIA and IIIB, and 20 (25%) were stages IVA and IVB. The mean neutrophil count was (4.5±2.2) ×109, lymphocyte count was (1.7±0.7) ×109 and platelet count was (239.2±68.9) ×109. The mean NLR was 3.1±2.1. The 25th, 50th and 75th NLR percentile were 1.7, 2.5 and 4.1 respectively. The mean PLR was 164.7±80.4 and the 25th, 50th and 75th PLR percentile were 120.2, 150.4 and 184.8 individually. Sixty-nine patients (87%) underwent open surgery and 10 patients (13%) with VATS. Of the patients given adjuvant therapy after surgery, 56 (71%) received radiotherapy and 33 (42%) received chemotherapy (10 cases received both radiotherapy and chemotherapy).
Full table
Disease-free survival
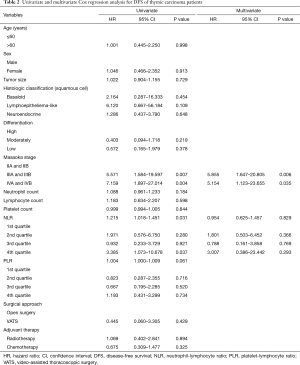
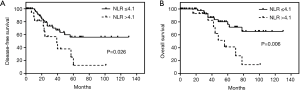
During the follow-up period, the 1-, 3-, and 5-year DFS rates were 78%, 57% and 44% respectively. In the Cox univariate regression analysis, both NLR, as a continuous variable (HR: 1.215, 95% CI: 1.018–1.451, P=0.031) and presence in the 4th NLR quartile (NLR >4.1, i.e., the 75th percentile), as a categorical variable (HR: 3.385, 95% CI: 1.073–10.678, P=0.037), and Masaoka stage IIIa and IIIb (HR =5.571; 95% CI: 1.584–19.597; P=0.007), IVa and IVb (HR =7.159; 95% CI: 1.897–27.014; P=0.004) were significantly associated with DFS. However, neither PLR (as a continuous variable, P=0.061) nor the PLR quartiles (as a categorical variable, P>0.05), neutrophil count (P=0.184), lymphocyte count (P=0.598) and platelet count (P=0.844) as well as age (P=0.998), sex (P=0.913), tumor size (P=0.729), histological classification (P>0.05), tumor differentiation (P>0.05), surgical approach (P>0.05) and adjuvant therapy (P>0.05) were not significantly associated with DFS. When analyzed by Cox multivariate regression analysis, only the Masaoka stage remained as an independent prognostic factor (IIIA and IIIB: HR =5.855; 95% CI: 1.647–20.805; P=0.006, IVA and IVB: HR =5.154; 95% CI: 1.123–23.655; P=0.035) (Table 2). Kaplan-Meier analysis found the highest NLR quartile (i.e., NLR >75th percentile) was associated with a significantly increased risk of recurrence or metastasis (P=0.026, log-rank test) (Figure 1A).
Full table
Overall survival
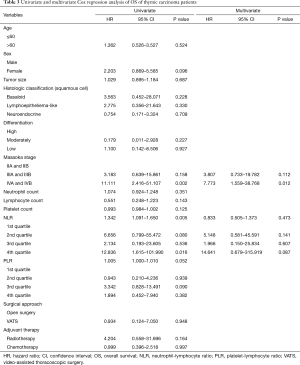
The Kaplan-Meier analysis indicated 1-, 3-, and 5-year OS rates of 96%, 79% and 60% respectively during follow-up. In the Cox univariate regression model, both NLR, as a continuous variable (HR: 1.342, 95% CI: 1.091–1.650, P=0.005) and the 4th NLR quartile (NLR >4.1, i.e., the 75th percentile), as a categorical variable (HR: 12.836, 95% CI: 1.615–101.990, P=0.016), and Masaoka stage IVa and IVb (HR =11.111; 95% CI: 2.416–51.107; P=0.002) was significantly associated with OS. However, age (P=0.524), sex (P=0.096), tumor size (P=0.687), histological classification (P>0.05), tumor differentiation (P>0.05), surgical approach (P>0.05), adjuvant therapy (P>0.05) and neither PLR (as a continuous variable, P=0.052) nor the PLR quartiles (as a categorical variable, P>0.05), neutrophil count (P=0.351), lymphocyte count (P=0.143) and platelet count (P=0.125) were not significantly associated with OS. Subsequently, the Cox multivariate analysis indicated that Masaoka stage (HR =7.773; 95% CI: 1.559–38.768; P=0.012) was the only variable found to be an independent predictor of OS (Table 3). The Kaplan-Meier analysis and log-rank tests found that patients in the 4th NLR quartile had a significantly shorter OS than other thymic carcinoma patients (P=0.006) (Figure 1B).
Full table
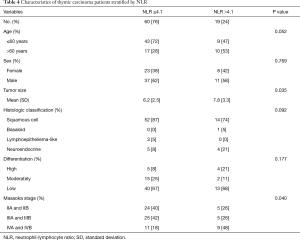
Further, compared with those in the lower NLR quartiles (NLR ≤4.1, i.e., the 75th percentile), the patients in the highest NLR quartile (NLR >4.1) who had high risk of recurrence or metastasis and death had larger tumor size (P=0.035) and more advanced Masaoka stages (P=0.040) (Table 4).
Full table
Discussion
In the present study of NLR, PLR and prognosis of thymic carcinoma, we found that increased preoperative NLR was significantly associated with tumor size, the Masaoka stage, disease recurrence or metastasis, and poor survival. Moreover, when patients were stratified by the 4th NLR percentile (NLR =4.1) found that the subgroup with NLR >4.1 had shorter DFS and OS than the subgroup with NLR at or below the 4.1 threshold. Furthermore, we found that Patients with an NLR >4.1 had bigger tumor volumes and more advanced Masaoka stages than those with an NLR ≤4.1. Although it was not an independent predictor of death and recurrence or metastasis, a high preoperative NLR of >4.1 was associated with decreased DFS and OS. The Masaoka staging system was an independent prognostic indicator of OS and DFS in these thymic carcinoma patients, which is consistent with previous reports. However, preoperative PLR and other variables, such as age, sex, tumor size, histologic classification, tumor differentiation, absolute white cells and platelet count, surgical approach and adjuvant therapy were not significantly associated with DFS and OS. Taking all these into consideration, the preoperative NLR can be an alternative prognostic marker for patients with thymic carcinoma and a supplementary for the Masaoka staging system. The 5-year DFS and the 5-year OS of thymic carcinoma patients after complete resection in our hospital seem to be better than those reported in other retrospective studies (3-5,20,21) (44% vs. an average of 40% and 60% vs. an average 42%, respectively).
Cancer-related inflammation has been shown to have adverse effects on cancer prognosis. The development and progression of cancer depends on a complex interaction of the tumor characteristics and the host inflammatory response, but many of the molecular and cellular mechanisms mediating this relationship remain unresolved (22-24). The host inflammatory response triggered by a tumor is complex and involves alterations in neuroendocrine metabolism, hormones and hematopoietic changes including the relative numbers of circulating white cells and platelets. Changes in protein and energy metabolism include loss of muscle protein and acute-phase responses including C-reactive protein (CRP) and albumin (25). In addition, the tumor microenvironment influences the invading white cells and platelets to promote angiogenesis, invasion, motility and viability (26,27). The NLR and PLR reflect the populations of circulating white cells and platelets and have clinical advantages of being inexpensive and routinely measured in perioperative practice. They are, inferior to some other markers of a cancer-related systemic inflammatory response, such as serum CRP and albumin, the Glasgow prognostic score (GPS) or modified GPS (mGPS) (28,29), but serum CRP levels and other markers are not routinely performed as part of the preoperative assessment of patients. Hence, the NLR and PLR plus the absolute neutrophil, lymphocyte and platelet counts were assessed in patients with thymic carcinoma who were retrospectively evaluated in our study.
Recently, several studies have shown that an elevated pretreatment NLR or PLR was associated with poor prognosis in patients with various cancers. Moreover, the majority of those studies showed that the prognostic value of NLR seemed to be superior to that of PLR (25). The increasing evidence demonstrates that pro-inflammatory cytokines play a significant role in the tumor host interaction, such as IL-1ra, IL-6, IL-7, IL-8, IL-12, IL-17 and MCP-1. These inflammatory cytokines may establish and keep a tumor microenvironment favoring aggressive tumor behavior. An elevated NLR is considered to be associated with elevated circulating concentrations of these cytokines, which offers an insight into mechanisms underlying an elevated NLR (30,31). Nevertheless, the cutoff points of NLR proposed in previous studies differ, including NLR thresholds of 2.3, 4.02 or 5 in gastric cancer (14,32,33), 0.38, 2.4 or 3 in colorectal cancer (15,34,35), 2.3 or 2.5 in NSCLC (10,36), and consensus has not been established. The heterogeneity of those studies may contribute to the different results. Eagerly, a future prospective study with large sample is needed to give a definitive cut-off value of NLR with good sensitivity and specificity. To the best of our knowledge, this is the first evaluation of the prognostic value of the NLR and PLR in patients with thymic carcinoma. We found that an NLR threshold of 4.1 could stratify patients by risk of recurrence or metastasis and death. Patients with an NLR >4.1 had shorter DFS and OS as well as bigger tumor volumes and more advanced Masaoka stages than those with an NLR ≤4.1. The results are consistent with the hypothesis that a greater preoperative NLR reflects an enhanced host inflammatory response to more aggressive tumors (9).
Inflammation is known to play an important role in the origin of many cancers, and cancer-related inflammation is a key determinant of patient outcome (22). For example, some colorectal cancers derive from ulcerative colitis, which is characterized by recurrent ulceration with chronic irritation and inflammation. Many markers of the systemic inflammatory response have been shown to be associated with prognosis of patients with colorectal cancer (37). However, the cause of thymic tumors is unknown, and thymic carcinoma does not arise as a result of obvious acute or chronic inflammation. For this reason, it seems to be very difficult to detect the traces of a cancer-related inflammation response in thymic carcinoma. In addition, given the rarity of thymic carcinoma, the small patient sample was enrolled in our study, which contributed to the bias of the study inevitably. All these factors may explain the difference in our study results compared with previous investigations conducted in a number of other cancers.
The limitations of this study include the small sample of clinical cases due to the rarity of thymic carcinoma, which may partially account for the negative outcome for the prognostic value of the NLR and PLR in thymic carcinoma. Secondly, it was a retrospective study that analyzed clinical data from only one institute, which may lead to inaccurate results. Thirdly, the mechanism of the association between increasing NLR and progression of thymic carcinoma is not fully clarified. In the future, we are looking forward to prospective studies with large patient samples that will be able to clarify the relationship between the NLR, PLR and other systemic inflammation-based markers and prognosis in thymic carcinoma patients as well as the mechanism of the association between cancer-related inflammatory markers and progression of thymic carcinoma.
Conclusions
The present study demonstrated that a preoperative NLR >4.1 was significantly associated with reduced DFS and OS as well as larger tumor size and more advanced Masaoka stages, but was not an independent predictor of DFS and OS in thymic carcinoma after complete resection. The Masaoka stage was an independent prognostic indicator for thymic carcinoma. However, the PLR was not significantly associated with survival in thymic carcinoma patients in our study. While the NLR is not independent prognostic factor for thymic carcinoma, it may be useful for counseling patients with respect to treatment options and possible outcomes, and may also supplement the Masaoka staging system.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of the Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
References
- Carter BW, Okumura M, Detterbeck FC, et al. Approaching the patient with an anterior mediastinal mass: a guide for radiologists. J Thorac Oncol 2014;9:S110-8. [Crossref] [PubMed]
- Greene MA, Malias MA. Aggressive multimodality treatment of invasive thymic carcinoma. J Thorac Cardiovasc Surg 2003;125:434-6. [Crossref] [PubMed]
- Masaoka A. Staging system of thymoma. J Thorac Oncol 2010;5:S304-12. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Eng TY, Fuller CD, Jagirdar J, et al. Thymic carcinoma: state of the art review. Int J Radiat Oncol Biol Phys 2004;59:654-64. [Crossref] [PubMed]
- Kondo K. Optimal therapy for thymoma. J Med Invest 2008;55:17-28. [Crossref] [PubMed]
- Forquer JA, Rong N, Fakiris AJ, et al. Postoperative radiotherapy after surgical resection of thymoma: differing roles in localized and regional disease. Int J Radiat Oncol Biol Phys 2010;76:440-5. [Crossref] [PubMed]
- Rajan A, Giaccone G. Chemotherapy for thymic tumors: induction, consolidation, palliation. Thorac Surg Clin 2011;21:107-14. viii. [Crossref] [PubMed]
- Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218-30. [Crossref] [PubMed]
- Zhang H, Xia H, Zhang L, et al. Clinical significance of preoperative neutrophil-lymphocyte vs platelet-lymphocyte ratio in primary operable patients with non-small cell lung cancer. Am J Surg 2015;210:526-35. [Crossref] [PubMed]
- Zhang WW, Liu KJ, Hu GL, et al. Preoperative platelet/lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumour Biol 2015;36:8831-7. [Crossref] [PubMed]
- Huang GQ, Zhu GQ, Liu YL, et al. Stratified neutrophil-to-lymphocyte ratio accurately predict mortality risk in hepatocellular carcinoma patients following curative liver resection. Oncotarget 2016;7:5429-39. [PubMed]
- Wong BY, Stafford ND, Green VL, et al. Prognostic value of the neutrophil-to-lymphocyte ratio in patients with laryngeal squamous cell carcinoma. Head Neck 2016;38 Suppl 1:E1903-8. [Crossref] [PubMed]
- Lian L, Xia YY, Zhou C, et al. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark 2015;15:899-907. [Crossref] [PubMed]
- Seong MK. Prognostic Inflammation Score in Surgical Patients with Colorectal Cancer. J Korean Med Sci 2015;30:1793-9. [Crossref] [PubMed]
- Huang J, Dahl DM, Dong L, et al. Preoperative Neutrophil-to-Lymphocyte Ratio and Neutrophilia Are Independent Predictors of Recurrence in Patients with Localized Papillary Renal Cell Carcinoma. Biomed Res Int 2015;2015:891045.
- Jia W, Wu J, Jia H, et al. The Peripheral Blood Neutrophil-To-Lymphocyte Ratio Is Superior to the Lymphocyte-To-Monocyte Ratio for Predicting the Long-Term Survival of Triple-Negative Breast Cancer Patients. PLoS One 2015;10:e0143061. [Crossref] [PubMed]
- Inoue D, Ozaka M, Matsuyama M, et al. Prognostic value of neutrophil-lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Jpn J Clin Oncol 2015;45:61-6. [Crossref] [PubMed]
- Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol 2015;10:1383-95. [Crossref] [PubMed]
- Wu JX, Chen HQ, Shao LD, et al. Long-term follow-up and prognostic factors for advanced thymic carcinoma. Medicine (Baltimore) 2014;93:e324. [Crossref] [PubMed]
- Omasa M, Date H, Sozu T, et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: the Japanese Association for Research on the Thymus Database Study. Cancer 2015;121:1008-16. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [Crossref] [PubMed]
- Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol 2004;4:641-8. [Crossref] [PubMed]
- McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 2014;16:717-27. [Crossref] [PubMed]
- Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149-63. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Proctor MJ, Horgan PG, Talwar D, et al. Optimization of the systemic inflammation-based Glasgow prognostic score: a Glasgow Inflammation Outcome Study. Cancer 2013;119:2325-32. [Crossref] [PubMed]
- Kinoshita A, Onoda H, Imai N, et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer 2012;107:988-93. [Crossref] [PubMed]
- Motomura T, Shirabe K, Mano Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol 2013;58:58-64. [Crossref] [PubMed]
- Kantola T, Klintrup K, Väyrynen JP, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer 2012;107:1729-36. [Crossref] [PubMed]
- Pan QX, Su ZJ, Zhang JH, et al. A comparison of the prognostic value of preoperative inflammation-based scores and TNM stage in patients with gastric cancer. Onco Targets Ther 2015;8:1375-85. [Crossref] [PubMed]
- Graziosi L, Marino E, De Angelis V, et al. Prognostic value of preoperative neutrophils to lymphocytes ratio in patients resected for gastric cancer. Am J Surg 2015;209:333-7. [Crossref] [PubMed]
- Del Prete M, Giampieri R, Loupakis F, et al. Prognostic clinical factors in pretreated colorectal cancer patients receiving regorafenib: implications for clinical management. Oncotarget 2015;6:33982-92. [PubMed]
- Mori K, Toiyama Y, Saigusa S, et al. Systemic Analysis of Predictive Biomarkers for Recurrence in Colorectal Cancer Patients Treated with Curative Surgery. Dig Dis Sci 2015;60:2477-87. [Crossref] [PubMed]
- Shimizu K, Okita R, Saisho S, et al. Preoperative neutrophil/lymphocyte ratio and prognostic nutritional index predict survival in patients with non-small cell lung cancer. World J Surg Oncol 2015;13:291. [Crossref] [PubMed]
- Leitch EF, Chakrabarti M, Crozier JE, et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer 2007;97:1266-70. [Crossref] [PubMed]


