Surgical embolectomy for acute massive pulmonary embolism: state of the art
Introduction
Venous thromboembolism (VTE) encompass both deep vein thrombosis (DVT) and pulmonary embolism (PE), representing in Western countries the third most frequent cardiovascular disease (after ACS and Stroke), and the third most common cause of hospital-related death. Every year, up to one million individuals are affected by DVT/PE, with a similar incidence in the USA and Europe (0.96–3.0 per 1,000 and 0.75–2.69 per 1,000, respectively) (1). It has been estimated that every year the number of VTE-related deaths is between 100,000 and 296,000 in the USA and 370,000 in Europe (2). Moreover, non-lethal PE may lead to chronic disease and disability (3). However, the exact incidence of VTE is unknown and difficult to determine because PE may remain asymptomatic and is often diagnosed after incidental finding. In fact, data are likely to be substantially higher since silent PE develops in up to 50% of patients with DVT and may remain undiagnosed for long (2). The severity of acute PE is assessed according to in-hospital or 30-day mortality in low-risk (LR), intermediate-risk (or submassive) and high-risk (or massive) acute PE. Among these, 7 out of 10 patients suffer from LR-PE and have preserved right ventricular function and absence of biomarkers of cardiovascular damage. Signs of right heart dysfunction and/or presence of biomarkers of cardiovascular damage, without persisting hypotension or shock, characterize the submassive EP form. Lastly, acute massive PE is the most severe form, characterized by signs of compromised respiratory or haemodynamic condition, with signs and symptoms of shock and a mortality rate that exceeds 20% irrespective of treatment. Early diagnosis is extremely important in massive PE since 70% of patients die within 1 hour from the onset of clinical signs of the disease because of a dramatic reduction of cardiac output finally leading to cerebrovascular complications and multiorgan failure (4).
Pathophysiology
PE compromises both gas exchange and circulation. In fact, both mechanical obstruction and PE-associated vasoconstriction, which is mediated by increased release of thromboxane A2 and serotonin, may increase RV afterload (5). Of note, pulmonary vascular resistance arises dramatically with the increasing of the clot burden. As a result, RV wall is stretched, triggering a compensatory activation of the neurohumoral axis that exerts inotropic and chronotropic effects. Unfortunately, this temporary adaptive mechanism increases the trophic demand of the myocardium and may lead to RV bowing to the interventricular septum, a phenomenon that reduces LV systemic output, further exacerbating haemodynamic instability and hypotension (6). These combined effects, lead to a drop of coronary perfusion and ischaemic RV suffering. The consequent reduction of RV output compromises LV pre-load and systemic blood pressure, setting a pathophysiologic degenerative loop that may lead to cardiogenic shock, which is the primary cause of death in severe PE. Acute massive PE is the most severe form, with mortality rates exceeding 20% irrespective of treatment. In fact, acute massive PE can ultimately result in sudden death secondary to massive obstruction of the pulmonary bed (approximately 10% of PE cases) (7). In detail, acute massive PE is characterized by hemodynamic instability, persistent hypotension, and cardiogenic shock. Moreover, acute massive PE causes acute cor pulmonale, a condition consistent with acute RV dilation and failure, hypokinesis, tachycardia, the presence of gallop rhythm, acute tricuspid regurgitation and signs of increased central venous pressure.
Diagnosis
Acute PE is often associated with poorly predictive signs e.g., chest pain, dyspnea, haemoptysis and, infrequently, syncope and arterial hypotension. Despite the low specificity and sensitivity of these, the use of largely accepted prediction rules and appropriate straightforward diagnostic algorithms may help the clinician in the evaluation of the likeliness of an ongoing PE. These principles are extensively revised in the 2014 ESC guidelines for the diagnosis and management of acute PE (8) and are hereafter summarized.
First, the clinician should classify patients with suspected PE into pre-test clinical categories that correspond to the probability that the ongoing pathology is actually confirmed to be acute PE. The Well’s (9) and revised Geneva (10) rules are useful tools that have been extensively validated and simplified to encourage their clinical routine utilization (11,12). These rules can provide a two-level score {PE unlikely [prevalence (p.) ≈12%] – PE likely} or three-level score [low-probability (LP: p. ≈10%), medium probability (MP: p. ≈30%) and high-probability (HP: p. ≈65%)]. Clearly, severely ill patients suffering from systemic shock or hypotension undergo a more rapid diagnostic algorithm (Figure 1) that is performed on urgently or emergently.
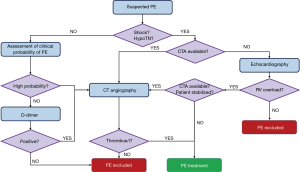
CT angiography (CTA) has become the method of choice for the diagnosis of PE, with a sensibility for a multi-detector device of 83% and a specificity of 96% (13). In fact, CTA allows a highly spatially and temporally resolved visualization of the arteries down to at least the segmental level (14). However, limited CTA availability and the intrinsic invasiveness of this technique may require different diagnostic tools to be used. Echocardiography may represent a useful non-invasive tool for haemodynamically unstable patients, detecting signs of RV overload and, eventually, direct imaging of the thromboemboli. Since RV overload is an aspecific sign, echocardiography should be used only to exclude PE for patients with haemodynamic instability. Moreover, in haemodynamically unstable patients without RV overload, echocardiography may reveal other possible causes of shock, e.g., heart tamponade, valvular dysfunction, aortic dissection, hypovolemia and left ventricular dysfunction. Conversely, if a further differential diagnosis is not immediately feasible and/or the patient is not stabilized, echocardiography may justify the use of emergency reperfusion (15). Due to its poor predictive value, echocardiography is not recommended for haemodynamically stable normotensive patients, which may undergo CTA in the case they are stratified as ‘high-probability PE’. Medium- and low-probability patients are tested for D-dimer, which is abundantly formed in case of concomitant activation of coagulation and fibrinolysis. Most ELISA or ELISA-derived D-dimer tests have a 95% sensibility and can, therefore, be used to exclude PE. On the contrary, a positive result should be further confirmed by CTA.
Management
Restoration of systemic pressure is necessary both for end-organ perfusion and to reverse RV ischemia. However, only small volumes (i.e., 500 mL) of fluid should be perfused to avoid deleterious compensatory overstretch responses that may further compromise RV function (16). In combination, a vasodilator such as norepinephrine and epinephrine are good choices to restore systemic blood pressure considering their positive inotropic effect. Once systemic blood pressure is restored, adding dobutamine can also exert beneficial effects on circulation. In fact, dobutamine can restore cardiac output and RV-PA coupling (17). Nevertheless, systemic vasodilation has been criticized for its lack of specificity. To directly improve lung perfusion and gas exchange, inhalation of nitric oxide has been proposed and triad in several trials (18,19). Respiratory failure caused by PE is best treated with rapid clot reduction (i.e., thrombolytics) without intubation and positive pressure ventilation. However, patients that undergo pre-surgical anesthesia or that show frank respiratory collapse may require intubation. Care should be paid to avoid that the positive intrathoracic pressure may worsen venous return. Smoothening of the transition to ventilation to a tidal volume not exceeding the 6–8 cc/kg lean body weight range may preserve the fragile preload-sensitive haemodynamic of RV failure (20).
To relieve obstruction and quickly restore blood flow in the pulmonary circulation, several options are available, including thrombolysis, percutaneous embolectomy, and surgical pulmonary embolectomy. The traditional treatment for PE consisted of anticoagulation with heparin therapy and watchful waiting, an approach that can be safely applied in most cases of stable PE. Unstable patients that require anticoagulation should be treated with unfractionated heparin (UFH), since this form has a short half-life and can be easily monitored (21). On the other hand, low molecular weight heparin (LMWH) should be preferred routinely, since it has a lower risk of inducing major bleeding and heparin-induced thrombocytopenia (22,23). Beside parenteral anticoagulation, vitamin K antagonists (VKA) should be administrated as soon as possible (24). However, non-vitamin K oral anticoagulants (NOACs), including rivaroxaban, apixaban, dabigatran, and edoxaban, showed to be non-inferior to traditional anticoagulant therapy and are associated with reduced bleeding risk and VTE recurrence (25).
Nevertheless, hemodynamic instability and evidence of RV failure may require more rapid intervention, such as thrombolysis, catheter-based therapies and/or surgical embolectomy. In fact, the early resolution of the obstruction in the pulmonary circulation immediately improves the patient’s hemodynamics, with a reduction in RV preload and resistance. Thrombolysis within the first week from the clinical onset of the symptoms and, best, within 48 hours was proved to be beneficial for the 90% of patients (26). Possible major complications include several forms of life-threatening bleeding and hemodynamic deterioration. Interestingly, the use of catheter-based technologies mitigates the intrinsic bleeding risk associated with systemic thrombolysis. A number of interventional options are available for patients with absolute contraindication to thrombolysis, including rheolytic thrombectomy with hydrodynamic catheter devices, rotational thrombectomy, suction thrombectomy and traditional thrombus fragmentation with pigtail or balloon catheter (27). Conversely, catheter-directed thrombolysis or pharmaco-mechanical thrombolysis are the preferred approaches for patients without absolute contraindications. In fact, local delivery may help to reduce the dosing and time of the treatment as well as the side effects, while improving its outcome (28). Moreover, augmented thrombolysis is achieved using catheter-based ultrasonic devices (e.g., EkoSOnic Endovascular System), which mechanically destroy the fibrin strands, in combination with the local infusion of the chosen fibrinolytic agent.
However, in one hand, thrombolysis and catheter thromboembolectomy can rapidly re-establish hemodynamic stability, but, on the other hand, these treatments may cause distal fragment embolization and hemorrhages.
Surgical pulmonary embolectomy is an option recommended in cases of high risk and cardiogenic shock, massive PE patients who cannot receive fibrinolysis, or remain unstable after its administration, submassive PE patients for whom thrombolysis is contraindicated or have failed and patients with right heart thrombi located close or straddling through a patent foramen ovale (29).
Traditionally, pharmacological options were preferred to surgical pulmonary embolectomy (SPE) because of the higher mortality rate and invasiveness of the latter. In detail, this approach had mortality rates ranging from 16% to 64% (30). Peri- and post-operative mortality has progressively decreased over the time, despite the ongoing use of pulmonary embolectomy as the last choice in very high-risk patients. The improvement of the surgical outcome generated new interest for pulmonary embolectomy as a viable treatment option in acute massive pulmonary embolism (AMPE) and resulted in an extension of the eligibility criteria for surgical embolectomy to those who are hemodynamically stable but show signs of impending right ventricular (RV) failure. In fact, several recent reports indicate that early mortality rates after surgical embolectomy have improved to 6–29% (31): Takahashi and al. reported a 12.5% in-hospital mortality rate for 24 patients undergoing embolectomy (32); Leacche et al. reported a series of 47 patients undergoing embolectomy with 6% of peri operative mortality rate (30). Other studies compared surgical versus medical therapy: e.g., Greelish et al. (33) recorded low mortality in 15 patients treated with surgical embolectomy (47% of them were unstable at presentation) compared with 88 patients undergoing medical treatment (unstable hemodynamic in 8% of them at presentation). Early mortality was almost equivalent (surgery 13% vs. medical 17%) but late mortality was lower at follow-up in surgical groups. A recent meta-analysis (34) shows the outcomes in 56 studies from 1965 to 2015, involving 1,579 patients who underwent 1,590 SPE operations. The authors highlight an in-hospital all-cause mortality of 26.3% and a long-term all-cause mortality rate of 6.5 deaths per 100 person-year. Among these patients, 1/3 had cardiac arrest before surgery, and 1/3 of operations required preoperative ECMO support. This study also shows how In-hospital mortality rates have fallen over years for patients undergoing SPE, particularly in centers where a great volume of SPEs is performed. These results may also reflect a better patient stratification, more rapid diagnostic pathways and protocols, and improved perioperative care.
Surgical technique
Before the advent of CPB, pulmonary embolectomy was performed under occlusion of the vena cava, with a quick incision in the pulmonary trunk, then removal of the embolus, and suturing of the pulmonary trunk. Nowadays, the surgical technique employs normothermic CPB using bicaval cannulation and aortic perfusion, but cardiac arrest is indeed not mandatory. In past years, deep hypothermic circulatory arrest has been widely used, but today this method is adopted only in specific cases, e.g., for the optimization of the surgical view in complete embolectomy in patients suffering from chronic thromboembolic pulmonary hypertension (35).
In the standard technique, after systemic anticoagulation is administrated, median sternotomy is performed and CPB is instituted. Then, the venae cavae are snared and the pulmonary trunk is incised in the proximity of the pulmonary valve. The location of the thrombus determines the extension and shape of the cut. In details, left trunk arteriotomy is usually performed in a hockey-stick fashion, while right pulmonary artery is incised between the aorta and the superior vena cava. The extraction of large thromboemboli in the proximal pulmonary artery may be facilitated by the insertion from the pulmonary trunk of forceps and a suction tube. However, the applicability of this extraction procedure is limited to directly visible thromboemboli at the level of the segmental pulmonary arteries (36). Many surgical techniques of thrombus extraction have been described aiming to refine the procedure at any point, e.g., setting minimally invasive alternative to conventional sternotomy, which has always been a significant barrier to the broad adoption of the surgical approach (37).
The opening of the bilateral pleura and peripheral clot extrusion through manual compression of the lungs have also been used. However, attention must be paid with patients treated with heparin to avoid mechanical injury of the arterial walls and lung parenchyma, which may cause endobronchial bleeding. In fact, squeezing of the lung may trigger critical lung bleeding in patients on thrombolytic therapy, which often leads the patient to death. Recently, Fukuda et al. (38) have reported that massive hemorrhage in patients under preoperative thrombolytic therapy may be managed infusing fresh frozen plasma and platelet precipitate. This procedure should be coupled with blocking of the involved bronchus with a balloon catheter in cases of arterial injury. Lastly, heparin should be reversed after the weaning of the CPB, which is often difficult to perform due to hypoxia. Interestingly, in a surgical scenario in which the right atrium is directly inspected for suspected clots, a sophisticated and original maneuver has been described by Zarrabi et al. (39). In details, a septal incision through the fossa ovalis is performed to get access to the left atrium. Then, the surgeon sequentially inserts into each pulmonary vein a cannula connected to the pump oxygenator and flushes for 60–80 seconds with a mean pressure of 15–17 mmHg. This pressure flushes retrogradely through the pulmonary veins clot and debris, which are then removed through pulmonary arteriotomy. The procedure lasts 5 minutes and, typically, it is possible to directly suture the pulmonary arteriotomy. Nevertheless, a bovine pericardial patch may be used in those patients whose pulmonary artery is particularly thin and fragile. When these efficient, time-saving procedures are operated, the patient can be weaned from CPB without any variation from the standard method.
Extracorporeal membrane oxygenation
Mechanical cardiopulmonary support is traditionally used for cardiothoracic surgery whenever cardiopulmonary bypass is temporarily required to facilitate circulation and oxygenation during the operation. Prolonged cardiopulmonary support is also used outside of the operating room in the intensive care unit to provide respiratory and/or hemodynamic assistance, a practice that is called ‘extracorporeal membrane oxygenation’ (ECMO). Two forms of ECMO are primarily utilized in the clinical practice: veno-arterial (VA) and veno-venous (VV). Both these forms can provide respiratory support by oxygenating and removing carbon dioxide (CO2) from the blood, but only VA ECMO provides hemodynamic support and is thus adopted in patients with cardiogenic shock. It is worth mentioning a third form of ECMO, called arterio-venous (AV) ECMO, which is limited to cases of poor blood flow to remove CO2. In general, ECMO should be considered as a bridge to recovery, while other therapeutic interventions are being performed and/or as an external support to facilitate the natural healing process. In fact, ECMO is generally indicated for patients suffering from any respiratory or cardiac failure that is potentially reversible when conventional therapies have failed (40). Clinical scenarios in which ECMO may be used include severe hypoxemic or hypercapnic respiratory failure despite ventilator optimization, cardiogenic shock refractory to treatment, and bridge to cardiac or lung transplantation. ECMO in any case in which there is a contraindication to anticoagulation (i.e., recent surgery, high bleeding risk, recent hemorrhagic stroke, etc.) since ECMO requires full heparinization to work without blocking the system. Other contraindications may include a poor baseline functional status, advanced age, neurologic dysfunction, severe obesity, unrecoverable condition, not a candidate for transplant or ventricular assist device, chronic organ dysfunction such as emphysema, cirrhosis, or renal failure, and prolonged cardiopulmonary resuscitation (CPR) without adequate perfusion of end organs.
In the setting of massive pulmonary embolism and hemodynamic failure, cardio-pulmonary support should be used according to the following principles:
- Reduction in PVR;
- Bridge to systemic or catheter-based thrombolysis, or surgery or catheter embolectomy;
- RV preload optimizations;
- Restoration of systemic blood pressure and RV coronary perfusion by optimizing afterload (vasoconstriction);
- Improvement in RV function;
Although most patients can be weaned from CPB after surgical pulmonary embolectomy, some patients with right ventricular failure need post-operative heart-lung assist. In massive PE, ECMO is an important therapeutic option for the hemodynamic stabilization of the patient before pulmonary embolectomy. However, the most severe complication is brain damage from prolonged hypoxemia and hypotension before surgery. Brain damage after pulmonary embolectomy is a critical concern, particularly after the long period of circulatory arrest that is required to perform the intervention. Rapid decision making about ECMO treatment is crucial to saving critically ill patients.
Another valuable option for right ventricle assistance is the Impella RP device (41), a 9-Fr catheter designed to reach the right atrium through a standard femoral vein insertion. This pump draws blood from an inlet that sits in the IVC and expels it directly into the pulmonary artery. This device can pump up to 4.0 L of blood and it has been studied and approved for the treatment of right heart failure and is based on the same technology of its predecessors (i.e., Impella CP and 5.0). These are percutaneously placed and augment left ventricular cardiac output in case of high-risk percutaneous coronary intervention and cardiogenic shock. To date, this device has not been yet approved for RV failure in the setting of PE, but it’s possible that it may have a role in future since it has been shown that both stable and unstable patients with PE who undergo pulmonary embolectomy may benefit from an IVC filter (42).
Conclusions
The outcomes of pulmonary embolism are highly dependent on the presence or absence of circulatory collapse, and very advanced cardiac condition as cardiac arrest and external massage. A multidisciplinary approach with rapid non-invasive diagnostics, proper risk stratification and availability of immediate surgical treatment are crucial to achieving superior results. Since many studies highlight higher in-hospital mortality among patients with preoperative cardiac arrest, SPE should be considered for patients before the development of advanced hemodynamic instability, and before progression to cardiogenic shock. In long-term studies, there is evidence that long-term cardiovascular and non-cardiovascular mortality rates are similar. Of note, PE is often related with other pathologies, and the patient survival depends often on the predisposing disease rather than the PE itself, as long as PE is properly treated. The increasing availability of ECMO and the increasing efforts for the standardization of this delicate surgical procedure has dramatically improved the post-operative outcome of SPE, suggesting that surgery is a valuable option in the treatment of PE with severe RV dysfunction or hemodynamic instability. There is a need to reeducate in the future, both medical and surgical trainees should be formed and/or updated on the role of SPE in the treatment of acute PE, particularly in those centers in which is available a surgical expertise in performing SPE. However, further new studies reporting the outcomes of pulmonary embolectomy are crucial to improve decision-making about the treatment protocols for AMPE and to increase awareness of surgical management options and coordination among the members of a well-trained multidisciplinary team.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol 2015;12:464-74. [Crossref] [PubMed]
- Fernandez MM, Hogue S, Preblick R, et al. Review of the cost of venous thromboembolism. Clinicoecon Outcomes Res 2015;7:451-62. [Crossref] [PubMed]
- Klok FA, van Kralingen KW, van Dijk AP, et al. Quality of life in long-term survivors of acute pulmonary embolism. Chest 2010;138:1432-40. [Crossref] [PubMed]
- Sekhri V, Mehta N, Rawat N, et al. Management of massive and nonmassive pulmonary embolism. Arch Med Sci 2012;8:957-69. [Crossref] [PubMed]
- Smulders YM. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res 2000;48:23-33. [Crossref] [PubMed]
- Mauritz GJ, Marcus JT, Westerhof N, et al. Prolonged right ventricular post-systolic isovolumic period in pulmonary arterial hypertension is not a reflection of diastolic dysfunction. Heart 2011;97:473-8. [Crossref] [PubMed]
- Belohlavek J, Dytrych V, Linhart A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol 2013;18:129-38. [PubMed]
- Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033-69, 69a-69k.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost 2000;83:416-20. [Crossref] [PubMed]
- Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006;144:165-71. [Crossref] [PubMed]
- Gibson NS, Sohne M, Kruip MJ, et al. Further validation and simplification of the Wells clinical decision rule in pulmonary embolism. Thromb Haemost 2008;99:229-34. [PubMed]
- Klok FA, Mos IC, Nijkeuter M, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med 2008;168:2131-6. [Crossref] [PubMed]
- Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317-27. [Crossref] [PubMed]
- Ghaye B, Szapiro D, Mastora I, et al. Peripheral pulmonary arteries: how far in the lung does multi-detector row spiral CT allow analysis? Radiology 2001;219:629-36. [Crossref] [PubMed]
- Kucher N, Luder CM, Dornhofer T, et al. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J 2003;24:366-76. [Crossref] [PubMed]
- Mercat A, Diehl JL, Meyer G, et al. Hemodynamic effects of fluid loading in acute massive pulmonary embolism. Crit Care Med 1999;27:540-4. [Crossref] [PubMed]
- Manier G, Castaing Y. Influence of cardiac output on oxygen exchange in acute pulmonary embolism. Am Rev Respir Dis 1992;145:130-6. [Crossref] [PubMed]
- Imanaka H, Miyano H, Takeuchi M, et al. Effects of nitric oxide inhalation after pulmonary thromboendarterectomy for chronic pulmonary thromboembolism. Chest 2000;118:39-46. [Crossref] [PubMed]
- Kline JA, Hall CL, Jones AE, et al. Randomized trial of inhaled nitric oxide to treat acute pulmonary embolism: The iNOPE trial. Am Heart J 2017;186:100-10. [Crossref] [PubMed]
- Ventetuolo CE, Klinger JR. Management of acute right ventricular failure in the intensive care unit. Ann Am Thorac Soc 2014;11:811-22. [Crossref] [PubMed]
- Raschke RA, Gollihare B, Peirce JC. The effectiveness of implementing the weight-based heparin nomogram as a practice guideline. Arch Intern Med 1996;156:1645-9. [Crossref] [PubMed]
- Cossette B, Pelletier ME, Carrier N, et al. Evaluation of bleeding risk in patients exposed to therapeutic unfractionated or low-molecular-weight heparin: a cohort study in the context of a quality improvement initiative. Ann Pharmacother 2010;44:994-1002. [Crossref] [PubMed]
- Stein PD, Hull RD, Matta F, et al. Incidence of thrombocytopenia in hospitalized patients with venous thromboembolism. Am J Med 2009;122:919-30. [Crossref] [PubMed]
- De Caterina R, Husted S, Wallentin L, et al. Vitamin K antagonists in heart disease: current status and perspectives (Section III). Position paper of the ESC Working Group on Thrombosis--Task Force on Anticoagulants in Heart Disease. Thromb Haemost 2013;110:1087-107. [Crossref] [PubMed]
- Bromley A, Plitt A. A Review of the Role of Non-Vitamin K Oral Anticoagulants in the Acute and Long-Term Treatment of Venous Thromboembolism. Cardiol Ther 2018;7:1-13. [Crossref] [PubMed]
- Meneveau N, Seronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest 2006;129:1043-50. [Crossref] [PubMed]
- Kuo WT, Gould MK, Louie JD, et al. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol 2009;20:1431-40. [Crossref] [PubMed]
- Jolly M, Phillips J. Pulmonary Embolism: Current Role of Catheter Treatment Options and Operative Thrombectomy. Surg Clin North Am 2018;98:279-92. [Crossref] [PubMed]
- Yavuz S, Toktas F, Goncu T, et al. Surgical embolectomy for acute massive pulmonary embolism. Int J Clin Exp Med 2014;7:5362-75. [PubMed]
- Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg 2005;129:1018-23. [Crossref] [PubMed]
- Sadaba JR, Greco E, Alvarez LA, et al. The surgical option in the management of acute pulmonary embolism. J Card Surg 2008;23:729-32. [PubMed]
- Takahashi H, Okada K, Matsumori M, et al. Aggressive surgical treatment of acute pulmonary embolism with circulatory collapse. Ann Thorac Surg 2012;94:785-91. [Crossref] [PubMed]
- Greelish JP, Leacche M, Solenkova NS, et al. Improved midterm outcomes for type A (central) pulmonary emboli treated surgically. J Thorac Cardiovasc Surg 2011;142:1423-9. [Crossref] [PubMed]
- Kalra R, Bajaj NS, Arora P, et al. Surgical Embolectomy for Acute Pulmonary Embolism: Systematic Review and Comprehensive Meta-Analyses. Ann Thorac Surg 2017;103:982-90. [Crossref] [PubMed]
- Trummer G, Berchtold-Herz M, Martin J, et al. Successful treatment of pulmonary hypertension with inhaled nitric oxide after pulmonary embolectomy. Ann Thorac Surg 2002;73:1299-301. [Crossref] [PubMed]
- Hui DS, McFadden PM. Contemporary Surgical Management of Acute Massive Pulmonary Embolism. In: Firstenberg MS, editor. Principles and Practice of Cardiothoracic Surgery. Rijeka: InTech; 2013. p. Ch. 17.
- Pasrija C, Shah A, Sultanik E, et al. Minimally Invasive Surgical Pulmonary Embolectomy: A Potential Alternative to Conventional Sternotomy. Innovations (Phila) 2017;12:406-10. [PubMed]
- Fukuda I, Daitoku K. Surgical Embolectomy for Acute Pulmonary Thromboembolism. Ann Vasc Dis 2017;10:107-14. [Crossref] [PubMed]
- Zarrabi K, Yarmohammadi H, Ostovan MA. Retrograde pulmonary embolectomy in massive pulmonary embolism. Eur J Cardiothorac Surg 2005;28:897-9. [Crossref] [PubMed]
- ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support Extracorporeal Life Support Organization, Version 1.4 August 2017 Ann Arbor, MI, USA.
- Cheung AW, White CW, Davis MK, et al. Short-term mechanical circulatory support for recovery from acute right ventricular failure: clinical outcomes. J Heart Lung Transplant 2014;33:794-9. [Crossref] [PubMed]
- Stein PD, Matta F, Lawrence FR, et al. Usefulness of Inferior Vena Cava Filters in Unstable Patients With Acute Pulmonary Embolism and Patients Who Underwent Pulmonary Embolectomy. Am J Cardiol 2018;121:495-500. [Crossref] [PubMed]


