Management of bladder neck stenosis and urethral stricture and stenosis following treatment for prostate cancer
Introduction
Bladder neck stenosis and urethral stricture are recognised complications of all treatments for prostate cancer. The majority of these are able to be managed with simple endoscopic interventions. Recalcitrant contractures are relatively rare overall, however these are associated with significant morbidity, often requiring multiple interventions with associated complications and impact upon quality of life. A small proportion of patients will have concomitant incontinence with intervention for their stricture, which must be considered in terms of further treatment with a urethral sling or artificial urinary sphincter (AUS). This review will focus on management of urethral stenosis and stricture disease following treatment for prostate cancer, including radical prostatectomy (RP), radiotherapy, cryotherapy and high intensity focused ultrasound (HIFU).
Nomenclature
This review uses an updated terminology to classify urethral strictures depending upon their anatomical location. The term stricture is used if the narrowing is in the part of the urethra that is surrounded by corpus spongiosum which includes fossa navicularis, penile and bulbar urethra. The term stenosis is used for narrowing in the membranous and prostatic urethra, bladder neck and post-prostatectomy anastomosis (1,2).
Background
Incidence
The Cancer of the Prostate Strategic Urologic Research Endeavour database (CaPSURE) is the largest cohort to date with 6,597 men undergoing treatment for prostate cancer, including watchful waiting, with a median follow-up of 2.7 years. Based on this data, the overall incidence of urethral stricture and stenosis treatment following prostate cancer therapy is 5.2% (3). True incidence of urethral stenosis or stricture disease is likely underestimated, as it is only identified when symptomatic post operatively, incidentally when undergoing catheterization for urodynamic evaluation, unrelated surgical or medical reasons or when presenting with late complications such as infections, stones, retention or renal failure. There is a higher risk of all urethral stricture disease in this patient population independent of prostate cancer intervention, with the incidence of strictures notably higher in men over the age of 50 (4), with 1.1% men with prostate cancer on watchful waiting noted to develop urethral strictures (3).
RP
The majority of strictures or stenoses post RP occur at the anastomosis, referred variably in the literature as bladder neck contracture or stenosis or as anastomotic stenosis or stricture and often are not differentiated from bulbo-membranous strictures (3). Reported rates in the literature range from 2.2% to 20.5% (5-7) subsequent to open radical prostatectomy (ORP), with contemporary data in the open cohorts reporting rates post-radical retropubic prostatectomy of 2.5–5.5% (8,9) and post-radical perineal prostatectomy of 3.8% (9). The laparoscopic and robotic cohorts report much lower rates of 0.2–3% (10-13). The CaPSURE database reported overall stricture and stenosis rates post-radical prostatectomy of 8.4% (3). The surveillance, epidemiology, and end results program (SEER) database has been utilised to compare laparoscopic and robotic RP with ORP, with reported stricture rates of 5.8% and 14% respectively (14).
The average time to presentation with urethral stenosis is 3.8–4.8 months, with the majority presenting within the first year (12,15). Radiation therapy is discussed below, but the risk of complications is significantly higher in multimodal therapy, with salvage prostatectomy estimated to have a post-operative risk of urethral stenosis of 26% (16).
Radiation therapy
Post radiation strictures are observed to occur 1–3 years after prostate cancer treatment and predominantly occur in the bulbomembranous urethra (>90%) (17-19). Incidence of urethral strictures as per the CaPSURE database post radiotherapy is estimated between 1.7–5.2% at median follow up of 2.7 years, depending upon treatment type, with a higher risk with combination therapy [brachytherapy (BT) + external beam radiotherapy (EBRT)] (3). The risk of stricture increases over time, with reported rates as high as 8.5% at 24–36 months (20) and 12% at 5 years (21). The incidence after ERBT is 1.7% (1–13%) for primary ERBT and 3–8.5% in the salvage setting (1,3). For BT the incidence is around 1.8% in the primary setting (3,22) and again higher in the salvage setting, at 7.5% (16). Recent studies revealed that the risk is dose dependent and is higher with high dose rate brachytherapy (HDR-BT) (11%) compared to LDR-BT (4%) (23). Incidence is highest overall after combination ERBT and BT (5.2–16%) (1-3).
Other modalities
Cryotherapy is associated with an incidence of urethral stricture or stenosis of 2.5–5.6% and seen mostly in the bladder neck and prostatic urethra (3,22,24). The risk after salvage cryotherapy is slightly higher at 10–11.7% (16,25). The incidence of urethral stenosis after HIFU is around 10% (1.8–40%) in the primary setting and 15-20% in salvage setting, mostly seen in the bladder neck and prostatic urethra (16,22,26,27).
Aetiology and pathophysiology
Surgical bladder neck stenosis and urethral stricture are the result of luminal constriction caused by tissue fibrosis. Any wound healing by primary intention undergoes wound contraction in the proliferative phase, driven by myofibroblasts, with further collagen remodeling during the maturation phase. As such, all wounds are prone to a degree of contraction, all the more important in the context of a luminal end-to-end anastomosis. Healing by secondary intention occurs when there is a loss of mucosal apposition, which may be the result of tissue ischemia secondary to tension, wound distraction secondary to haematoma and foreign materials such as clips or poor technical apposition with resultant urinoma. Healing by secondary intention is driven by fibroblast differentiation into myofibroblasts, with increased collagen deposition. This is known to have a much greater risk of wound contracture and hypertrophic scarring, with resultant luminal narrowing.
The mechanism of action of radiation therapy is to cause DNA damage and free radical formation, with resultant apoptosis. This activates pro-inflammatory and pro-fibrotic cytokines leading to tissue and vascular injury (endarteritis), which leave the tissues poorly oxygenated, with increased collagen deposition, tissue contraction and scar formation (28,29). Over time, this results in progressive obliterative endarteritis with tissue necrosis and fibrosis (30). As a consequence, radiation induced strictures tend to present later than surgical stenoses and in a more insidious fashion, up to 2–3 years post treatment (3). Cryotherapy uses freezing temperatures to cause protein denaturation with apoptosis as a result of cell membrane rupture. HIFU uses high temperatures to again cause protein denaturation with immediate coagulative necrosis. The mechanism of action in stricture formation of these two therapies is in a similar vein to that of radiation, with tissue injury resulting in pro-inflammatory and pro-fibrotic cytokines and subsequent tissue contraction and scar formation.
Risk factors for formation of urethral stenosis and stricture
Post-radical prostatectomy
Patient factors, which increase the risk of stricture or stenosis formation, include age, obesity, smoking, diabetes mellitus, hypertension, cardiac disease and renal disease (3,31,32). Surgical predictors include previous transurethral resection of prostate (TURP), open surgery, prostate specific antigen (PSA) recurrence, intraoperative blood loss, operative time, post-operative urinary leak and post-operative retention (7,31). Certainly, the reported rates of urethral stenosis are significantly lower in the robotic or laparoscopic cohort, which would suggest that technique has a significant effect (33). In an analysis of risk factors for bladder neck stenosis formation, Sandhu et al described surgical technique as the strongest predictor with a hazard ratio of 0.11 for laparoscopic versus open surgery (32). In an ORP, most bladder neck repairs are undertaken with mucosal eversion on the bladder side, a longitudinal ‘racket handle’ at 6 o’clock to reduce the opening if required and anastomosed by interrupted circumferential sutures. These sutures are tied without visual cues per se and, particularly with the more technically challenging posterior sutures, there is a risk that the sutures may not snug down with appropriate tension or mucosal apposition, or that they may indeed “pull through”. Robotically or laparoscopically, there is no question that visual acuity is significantly improved. The anastomosis is performed using a continuous suture with a parachute technique, with no mucosal eversion.
Radiotherapy
Risk factors include age, obesity, hypertension, diabetes, previous TURP (15% vs. 6% without TURP), longer follow-up, higher radiation dose, HDR-BT, adjuvant RT and combination with BT (1,3,30,34). Delaying adjuvant RT for more than 9 months after RP may decrease stricture formation, however this is at the expense of an increase in cancer-specific mortality (34). Zelefsky found that intensity modulated RT (IMRT) increases the risk of late urinary toxicities including urethral stricture compared to 3-D conformal RT, but with lower rectal toxicity (17). However, a recent review found no difference in urethral stricture between 3-dimensional conformal radiotherapy (3D-CRT) and IMRT (35). Similar to ERBT, BT strictures affect the bulbomembranous urethra in the majority of cases, which could be due to a “hot spot” in the distal bulbar urethra (23) or due to caudal needle shifting in patients receiving HDR-BT (30), although Hindson found no relation between needle shifting and stricture incidence (36). A prospective, matched-pair analysis by Diez found no association between urethral stricture incidence and urethral dosimetry in patients receiving HDR-RT, however the number of events was too small to draw a definitive conclusion (37).
Studies are relatively limited looking at risk factors for stricture and stenosis in cryotherapy and HIFU. With cryotherapy, there have been recent technical refinements in the form of using a urethral warming catheter and real-time ultrasound-guided monitoring of freezing in an attempt to reduce complications, suggesting a reduction in the incidence of urethral sloughing and stenosis (22). However, no protective effect of urethral warming was found in other studies (38).
Management
It is often difficult to ascertain true efficacy and longevity of interventions for both responsive and recalcitrant stricture disease due to low patient numbers, a lack of randomized controlled trials, with predominantly retrospective studies and relatively short follow up. Overall, around 25–30% of patients will be recalcitrant, with more than three unsuccessful attempts at endoscopic procedures (31). Treatment of any stenosis or stricture should be individualised to the patient, taking into account overall patient health and patient preference. Considerations include the type of cancer treatment undertaken, the wound healing capacity and ability to support tissue transfer; the stricture or stenosis position, length and degree of luminal obliteration; the relation to the sphincter mechanism and underlying sphincter function and the functional status of the bladder, including detrusor overactivity and functional bladder capacity.
Simple and endoscopic intervention
The majority of surgical anastomotic stenoses (58%) respond to simple urethral dilatation or visual urethrotomy (31). This includes office-based techniques such as passage of urethral sounds or filiform followers and is often supplemented with a regime of clean intermittent self-catheterization (CISC). This correlates with the SEER data, which suggests that patients with BOO post-treatment for prostate cancer, 44% of patients will require more than one procedure (39). Park et al. demonstrated post-retropubic radical prostatectomy (RRP) patients with anastomotic stenoses who underwent dilatation followed by CISC, was successful in 92% at 1 year follow up, although it must be noted 27% required two or more procedures (40). They suggested that men with hypertrophic scars were at greater risk of anastomotic stricture, suggesting pathological wound healing.
More common practice in contemporary series of anastomotic stenoses is formal endoscopic evaluation with visual urethrotomy, in the form of cold Collins knife, monopolar or bipolar cutting currents and, in more recent times, lasers such as Holmium. Cold knife incision of the bladder neck is associated with good outcomes, Giannarini et al. demonstrating 73% success with a single incision and 100% success with repeated incision in a group of 46 patients with 48 months median follow up (15). There is little difference noted between monopolar and bipolar BNI, with outcomes from deep cutting current incisions in recurrent contractures reported with success rates of 72% and 89% after one and two procedures respectively (41). Urethral balloon dilators have been described in conjunction with BNI by the same group, with success in 72% after one and 86% after two procedures, interestingly not dissimilar results from BNI alone (42). Overall, the American Urological Association guidelines suggest that dilatation, BNI or transurethral resection will be successful in 50–80% post-prostatectomy patients (43).
After radiation, urethral dilatation and direct visual internal urethrotomy (DVIU) carry a high recurrence rate of up to 50% within 16–60 months (1) and if repeated, may increase stricture complexity and time to curative urethroplasty. Some studies still recommend dilatation or DVIU as the initial step in managing radiation-induced strictures (1,22) while other studies reserve endoscopic treatment only for patients who are unwilling or unable to undergo more invasive curative procedures (2,30,38,44). In Liberman’s review, the need for repeat procedures was significantly higher in the radiotherapy group (EBRT + BT), with a 68% higher likelihood of retreatment compared with RRP (39). Channeling TURP may be required for patients who develop retention following BT, cryotherapy or HIFU, keeping in mind the higher incontinence rate afterwards (1).
Steroids, with the most described agent triamcinolone (40 mg) (45), work by enhancing collagenase activity, act to break down peptide bonds in collagen to allow tissue remodeling. Eltahawy et al. evaluated 24 patients with recurrent or resistant bladder neck stenoses post-RRP who underwent Holmium laser BNI with injection of triamcinolone, with a success rate of 83% at 24 months (46).
Mitomycin C (MMC) is perhaps one of the most common injectable agents utilized in the prevention of BNC recurrence. Mitomycin has been used successfully for over 50 years in prevention of recurrent glaucoma, pterygium, post-surgical aerodigestive stenosis and in prevention of scarring post excision of keloid scars. The mechanism of action is to modulate and prevent scar formation by crosslinking DNA and preventing the proliferation of fibroblasts. Doses range from 0.1 to 10 mg (47). Vanni et al. retrospectively reviewed 18 patients with recurrent BNC, who underwent cold knife BNI and MMC injection and reported stabilization of the BNC in 72% after a single intervention and 89% after 2 interventions, with a median follow-up of 12 months and minimal morbidity reported (48). Lyon et al. performed a similar retrospective analysis of 13 patients, with stabilization in 62% (49). The TURNS study group looked retrospectively at 66 patients of whom 80% had previously failed a dilatation or BNI. These patients were treated with BNI and MMC; they found that the response rate was similar to that of BNI alone, with 58% stabilized after a single BNI and MMC injection and 75% overall after a second BNI and MMC (50). In addition, they identified a 7% rate of severe adverse events, including osteitis pubis, necrosis of the trigone and formation of rectourethral fistula.
The use of injectables in the treatment of anterior urethral strictures is controversial due to the vascularity of the corpus spongiosum, with a higher risk of systemic absorption and a theoretical reduction in the local availability and efficacy. A double-blind, placebo controlled randomized trial of anterior urethral strictures undergoing DVIU compared triamcinolone with sterile water, however while there was a delay in stricture recurrence of 4–5 months, there was no significant difference in overall rates of stricture recurrence (51), consistent with a meta-analysis of steroids as an adjunct to internal urethrotomy (52). Mazdak et al. undertook two prospective randomized controlled trials to determine if internal urethrotomy for anterior urethral strictures with submucosal injection of triamcinolone (45) or MMC (47) was more effective than internal urethrotomy alone. Both trials demonstrated a reduction in stricture recurrence rates with triamcinolone (21.7%) and MMC (10%) compared with matched control groups (50%, 50% respectively). It is worth noting that the numbers were small (50 and 40 patients) and the mean follow up was relatively short at 13 months and 6 months respectively. Triamcinolone, MMC and hyaluronidase combined had success rates of 80.6% at 14-month follow-up (53).
There are a number of novel scar-modulating agents that may be considered in the future as potential candidates for prevention of recurrent stricture disease. Xiaflex is a collagenase Clostridium histolyticum, which acts in a similar manner to corticosteroids, acting to break down peptide bonds between collagen molecules. It currently is approved for use in Dupuytren’s contracture and Peyronie’s disease, however has been used in animal models of urethral stricture with promising results (54).
Urethral stents
The UroLume urethral stent was proposed as a means to stabilize recurrent urethral strictures without the need for indwelling or intermittent catheterization or urinary diversion and was proposed in conjunction with an AUS for subsequent stress urinary incontinence (SUI). It was designed as a long-term stent, which would over time become incorporated into the urethral wall. Early results at 3 months were promising in terms of outcomes (55), however longer term studies demonstrated high complication and revision rates (56), including an overall failure rate of 24% (57) and poor long term satisfaction (58). Complications related to stent ingrowth, migration and encrustation resulted in at least 2 endoscopic interventions in 50% of patients, with an increased risk of concomitant AUS erosion in 35%. As such, Urolume is no longer recommended in this subset of patients.
More recently, temporary urethral stents such as the Memokath or the Allium have been proposed as a means for stabilizing urethral strictures. There is very little prospective data, however, as to outcomes, particularly in the setting of post-radical prostatectomy stricture disease. Adamakis et al. placed an Allium stent for a period of 6 months prior to removal, with subsequent placement of an AUS 6 months later, if the patients remained asymptomatic. The patient group were men with severe incontinence subsequent to intervention for previous, now recurrent, bladder neck contracture. The stricture recurrence rate was reported at 7%, however there was only 12 month follow up post-sphincter placement with a very small volume of patients, 14 in total (59). The Memokath, a thermos expandable stent, has been used particularly in spinal cord patients with detrusor sphincter dyssynergia and for bladder outlet obstruction. Yoon et al. examined 12-month placement of Memokath stents for anterior urethral strictures, and while they reported it as an effective therapeutic option, the majority of patients required further dilatations and 18% required further transurethral resection (60).
Open, laparoscopic and robotic management of refractory bladder neck stenosis
Refractory stricture disease provides quite the challenge to urologists and treatment must take into account both patient factors, including comorbidities, previous interventions, complications and surgical factors, including procedure-associated morbidity and surgical expertise (43). Certainly complex reconstruction of the bladder neck is not a commonly performed procedure and would generally be referred to a tertiary centre with reconstructive surgical experience.
Bladder neck reconstruction has been described in various fashions in small patient groups, including open abdominal, abdominoperineal and perineal approaches with high success rates (70–100%), however this was reported in only very small experienced with reconstruction (61-63). With advances in laparoscopic and robotic techniques, this is an area of constant evolution. Techniques including robotic assisted YV-plasty of the bladder neck (64) have been described in case reports and demonstrate early promise, however long term follow-up is required.
Post-prostatectomy incontinence in the setting of stricture disease
Park et al. demonstrated a significantly increased risk of stress incontinence in those with anastomotic stricture compared with controls at 1 year follow up (46.1% vs. 12.5%) (40), a well-recognized association (65). Post-prostatectomy SUI with concurrent post-operative bladder neck stenosis or urethral stricture may be managed in either a synchronous (65) or two-stage fashion, with management and stabilization of the narrowing followed by implantation of an AUS (66). In those with a recurrent or particularly dense narrowing, a two-stage approach would be generally recommended due to the increased risk of erosion with instrumentation post AUS insertion. The complication rate for artificial sphincters is higher after radiotherapy, particularly in the salvage setting (RP + EBRT), compared with RP alone (67). The alternative in the post-radical prostatectomy patient is the use of male sling, particularly if the volume of incontinence is low, although they have a high risk of persistent incontinence and complications following radiotherapy (68,69).
Open reconstruction of anterior urethral strictures
Options for treatment of anterior urethral strictures include excision and primary anastomosis (EPA) or substitution urethroplasty with graft and/or flap. Mundy and Andrich addressed the shortcomings of treatment of bulbomembranous strictures subsequent to RP, as few authors distinguish management from that of bladder neck stenosis (4). They proposed that surgical treatment should be tailored according to stricture length and sphincter involvement. If the sphincter is involved the initial management is dilatation to preserve the sphincter. EPA is used if dilatation fails but EPA in this setting is associated with high risk of incontinence and the patient may need an AUS later on (22). For short sub-sphincteric strictures, a non-transecting anastomosis or patch repair is preferred to avoid ischemia. For longer sub-sphincteric strictures, DVIU or dilatation has a low success rate and urethroplasty should be offered, using a dorsal onlay buccal mucosal graft (BMG) (4,43).
Surgical treatment of urethral stricture or stenosis after radiotherapy is challenging and radiation therapy has been identified as an independent risk for failure of urethroplasty (3,70). Urethral strictures after RT involve the bulbo-membranous area in more than 90% of cases (22). Strictures after ERBT are shorter and non-obliterative compared to those after BT (4). The surgical choice depends on stricture length, sphincter involvement, the degree of surrounding fibrosis and bladder capacity (4,22). In general, EPA is the treatment of choice for short strictures (less than 2–3 cm) while substitution urethroplasty by using BMG or flap (penile or perineal skin or gracilis muscle) is preferred for longer strictures when tension-free anastomosis is difficult to obtain (43). The choice between graft (ventral or dorsal only) or flap depends on surgeon preference. Some surgeons use grafts since they are harvested from a non-irradiated tissue while other surgeons prefer flaps especially when the stricture is long, or when in association with radiation changes, or when the degree of fibrosis is severe that may risk failure of graft uptake.
Studies published from 2011–2016 that addressed surgical treatment of radiation-induced strictures and their side effects have been reviewed and summarized in Table 1. The results are not directly comparable due to differences in the definition of success and recurrence, inclusion criteria, follow-up time, surgeon experience and preference. EPA performed in 183 patients had a 70–97% success rate within 3–4 years (70,72,75,76). BMG with or without gracilis muscle flap was done in 70 patients and had a success rate of 71–80% within 2–4 years (70,73,74,76). Flap urethroplasty was performed in 10 patients and had a success rate that varied between 50–100% within 2–4 years (70,71,76). Patient should be counseled that new onset urinary incontinence is seen in 5–35% after surgery and may require further intervention.
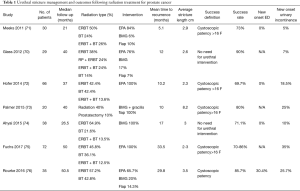
Full table
The end-stage bladder and devastated outlet
The combination of sphincteric damage and recurrent stricture or stenosis, after multiple failed attempts at repair of both, the so called ‘devastated outlet’ is difficult to rectify and has little existing literature to support appropriate management pathways (77,78). Similarly, the end stage bladder is often seen subsequent to radiotherapy. Treatment of prostate cancer is in constant evolution, however the current trend towards multimodal therapy for treatment of locally advanced disease or the use of adjuvant radiation for high risk features or PSA recurrence, is not without a significant increase in not only the complications but also the complexity of managing these complications.
The majority of patients in this setting, particularly if elderly, are often managed with suprapubic urinary diversion. Bladder preservation, with closure of the bladder neck and continent vesicostomy, with types of continence mechanisms including appendicovesicostomy, Mitrofanoff or intussuscepted ileal channels, are potential options, however the majority of evidence is based on the paediatric population and there is very limited published experience in men post-treatment for prostate cancer. Certainly there is some evidence that omental interposition at the bladder neck reduces the risk of recanalization and fistula formation and there is a suggestion that bladder augmentation, particularly in the post-radiotherapy group, will allow a low-pressure reservoir (79,80). Urinary diversion, continent or incontinent, with or without cystectomy (or cystoprostatectomy), is much more involved and considered the ‘end of the line’.
Conclusions
Most men with an initial presentation of bladder neck stenosis or urethral stricture subsequent to treatment for prostate cancer, especially without radiation, will often respond to simple endoscopic measures. In those men with recalcitrant disease, the chance of stabilization decreases with each intervention, and management is often more difficult involving major pelvic reconstructive surgery which carry higher risk of complications and associated morbidity. Significant urinary incontinence requiring second stage implantation of the artificial urinary sphincter is often required. Suprapubic urinary diversion is a last resort for those stenoses which are un-reconstructable, but yet do offer an improved quality of life for these most difficult of cases.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen ML, Correa AF, Santucci RA. Urethral Strictures and Stenoses Caused by Prostate Therapy. Rev Urol 2016;18:90-102. [PubMed]
- Khourdaji I, Parke J, Chennamsetty A, et al. Treatment of Urethral Strictures from Irradiation and Other Nonsurgical Forms of Pelvic Cancer Treatment. Adv Urol 2015;2015:476390. [Crossref] [PubMed]
- Elliott SP, Meng MV, Elkin EP, et al. Incidence of urethral stricture after primary treatment for prostate cancer: data From CaPSURE. J Urol 2007;178:529-34. [Crossref] [PubMed]
- Mundy AR, Andrich DE. Urethral strictures. BJU Int 2011;107:6-26. [Crossref] [PubMed]
- Carlsson S, Nilsson AE, Schumacher MC, et al. Surgery-related complications in 1253 robot-assisted and 485 open retropubic radical prostatectomies at the Karolinska University Hospital, Sweden. Urology 2010;75:1092-7. [Crossref] [PubMed]
- Kundu SD, Roehl KA, Eggener SE, et al. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol 2004;172:2227-31. [Crossref] [PubMed]
- Kao TC, Cruess DF, Garner D, et al. Multicenter patient self-reporting questionnaire on impotence, incontinence and stricture after radical prostatectomy. J Urol 2000;163:858-64. [Crossref] [PubMed]
- Erickson BA, Meeks JJ, Roehl KA, et al. Bladder neck contracture after retropubic radical prostatectomy: incidence and risk factors from a large single-surgeon experience. BJU Int 2009;104:1615-9. [Crossref] [PubMed]
- Gillitzer R, Thomas C, Wiesner C, et al. Single center comparison of anastomotic strictures after radical perineal and radical retropubic prostatectomy. Urology 2010;76:417-22. [Crossref] [PubMed]
- Breyer BN, Davis CB, Cowan JE, et al. Incidence of bladder neck contracture after robot-assisted laparoscopic and open radical prostatectomy. BJU Int 2010;106:1734-8. [Crossref] [PubMed]
- Parihar JS, Ha YS, Kim IY. Bladder neck contracture-incidence and management following contemporary robot assisted radical prostatectomy technique. Prostate Int 2014;2:12-8. [Crossref] [PubMed]
- Msezane LP, Reynolds WS, Gofrit ON, et al. Bladder neck contracture after robot-assisted laparoscopic radical prostatectomy: evaluation of incidence and risk factors and impact on urinary function. J Endourol 2008;22:97-104. [Crossref] [PubMed]
- Box GN, Ahlering TE. Robotic radical prostatectomy: long-term outcomes. Curr Opin Urol 2008;18:173-9. [Crossref] [PubMed]
- Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA 2009;302:1557-64. [Crossref] [PubMed]
- Giannarini G, Manassero F, Mogorovich A, et al. Cold-knife incision of anastomotic strictures after radical retropubic prostatectomy with bladder neck preservation: efficacy and impact on urinary continence status. Eur Urol 2008;54:647-56. [Crossref] [PubMed]
- Parekh A, Graham PL, Nguyen PL. Cancer control and complications of salvage local therapy after failure of radiotherapy for prostate cancer: a systematic review. Semin Radiat Oncol 2013;23:222-34. [Crossref] [PubMed]
- Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:1124-9. [Crossref] [PubMed]
- Merrick GS, Butler WM, Wallner KE, et al. Risk factors for the development of prostate brachytherapy related urethral strictures. J Urol 2006;175:1376-80. [Crossref] [PubMed]
- Sullivan L, Williams SG, Tai KH, et al. Urethral stricture following high dose rate brachytherapy for prostate cancer. Radiother Oncol 2009;91:232-6. [Crossref] [PubMed]
- Benoit RM, Naslund MJ, Cohen JK. Complications after prostate brachytherapy in the Medicare population. Urology 2000;55:91-6. [Crossref] [PubMed]
- Zelefsky MJ, Wallner KE, Ling CC, et al. Comparison of the 5-year outcome and morbidity of three-dimensional conformal radiotherapy versus transperineal permanent iodine-125 implantation for early-stage prostatic cancer. J Clin Oncol 1999;17:517-22. [Crossref] [PubMed]
- Herschorn S, Elliott S, Coburn M, et al. SIU/ICUD Consultation on Urethral Strictures: Posterior urethral stenosis after treatment of prostate cancer. Urology 2014;83:S59-70. [Crossref] [PubMed]
- Mohammed N, Kestin L, Ghilezan M, et al. Comparison of acute and late toxicities for three modern high-dose radiation treatment techniques for localized prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:204-12. [Crossref] [PubMed]
- Rodríguez SA, Arias Fúnez F, Bueno Bravo C, et al. Cryotherapy for primary treatment of prostate cancer: intermediate term results of a prospective study from a single institution. Prostate Cancer 2014;2014:571576. [Crossref] [PubMed]
- Kvorning Ternov K, Krag Jakobsen A, Bratt O, et al. Salvage cryotherapy for local recurrence after radiotherapy for prostate cancer. Scand J Urol 2015;49:115-9. [Crossref] [PubMed]
- Uchida T, Tomonaga T, Kim H, et al. Improved outcomes with advancements in high intensity focused ultrasound devices for the treatment of localized prostate cancer. J Urol 2015;193:103-10. [Crossref] [PubMed]
- Ahmed HU, Zacharakis E, Dudderidge T, et al. High-intensity-focused ultrasound in the treatment of primary prostate cancer: the first UK series. Br J Cancer 2009;101:19-26. [Crossref] [PubMed]
- Stone HB, Coleman CN, Anscher MS, et al. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol 2003;4:529-36. [Crossref] [PubMed]
- Gomes CM, Nunes RV, Tse V. Pelvic irradiation and its effects on the lower urinary tract: a literature review. Curr Bladder Dysfunct Rep 2015;10:295-302. [Crossref]
- Moltzahn F, Dal Pra A, Furrer M, et al. Urethral strictures after radiation therapy for prostate cancer. Investig Clin Urol 2016;57:309-15. [Crossref] [PubMed]
- Borboroglu PG, Sands JP, Roberts JL, et al. Risk factors for vesicourethral anastomotic stricture after radical prostatectomy. Urology 2000;56:96-100. [Crossref] [PubMed]
- Sandhu JS, Gotto GT, Herran LA, et al. Age, obesity, medical comorbidities and surgical technique are predictive of symptomatic anastomotic strictures after contemporary radical prostatectomy. J Urol 2011;185:2148-52. [Crossref] [PubMed]
- Webb DR, Sethi K, Gee K. An analysis of the causes of bladder neck contracture after open and robot-assisted laparoscopic radical prostatectomy. BJU Int 2009;103:957-63. [Crossref] [PubMed]
- Kowalczyk KJ, Gu X, Nguyen PL, et al. Optimal timing of early versus delayed adjuvant radiotherapy following radical prostatectomy for locally advanced prostate cancer. Urol Oncol 2014;32:303-8. [Crossref] [PubMed]
- Ballek NK, Gonzalez CM. Reconstruction of radiation-induced injuries of the lower urinary tract. Urol Clin North Am 2013;40:407-19. [Crossref] [PubMed]
- Hindson BR, Millar JL, Matheson B. Urethral strictures following high-dose-rate brachytherapy for prostate cancer: analysis of risk factors. Brachytherapy 2013;12:50-5. [Crossref] [PubMed]
- Díez P, Mullassery V, Dankulchai P, et al. Dosimetric analysis of urethral strictures following HDR (192)Ir brachytherapy as monotherapy for intermediate- and high-risk prostate cancer. Radiother Oncol 2014;113:410-3. [Crossref] [PubMed]
- Chi AC, Han J, Gonzalez CM. Urethral strictures and the cancer survivor. Curr Opin Urol 2014;24:415-20. [Crossref] [PubMed]
- Liberman D, Jarosek S, Virnig BA, et al. The Patient Burden of Bladder Outlet Obstruction after Prostate Cancer Treatment. J Urol 2016;195:1459-63. [Crossref] [PubMed]
- Park R, Martin S, Goldberg JD, et al. Anastomotic strictures following radical prostatectomy: insights into incidence, effectiveness of intervention, effect on continence, and factors predisposing to occurrence. Urology 2001;57:742-6. [Crossref] [PubMed]
- Ramirez D, Zhao LC, Bagrodia A, et al. Deep lateral transurethral incisions for recurrent bladder neck contracture: promising 5-year experience using a standardized approach. Urology 2013;82:1430-5. [Crossref] [PubMed]
- Ramirez D, Simhan J, Hudak SJ, et al. Standardized approach for the treatment of refractory bladder neck contractures. Urol Clin North Am 2013;40:371-80. [Crossref] [PubMed]
- Wessells H, Angermeier KW, Elliott S, et al. Male Urethral Stricture: American Urological Association Guideline. J Urol 2017;197:182-190. [Crossref] [PubMed]
- Milose JC, Gonzalez CM. Urethroplasty in radiation-induced strictures. Curr Opin Urol 2015;25:336-40. [PubMed]
- Mazdak H, Izadpanahi MH, Ghalamkari A, et al. Internal urethrotomy and intraurethral submucosal injection of triamcinolone in short bulbar urethral strictures. Int Urol Nephrol 2010;42:565-8. [Crossref] [PubMed]
- Eltahawy E, Gur U, Virasoro R, et al. Management of recurrent anastomotic stenosis following radical prostatectomy using holmium laser and steroid injection. BJU Int 2008;102:796-8. [Crossref] [PubMed]
- Mazdak H, Meshki I, Ghassami F. Effect of mitomycin C on anterior urethral stricture recurrence after internal urethrotomy. Eur Urol 2007;51:1089-92. [Crossref] [PubMed]
- Vanni AJ, Zinman LN, Buckley JC. Radial urethrotomy and intralesional mitomycin C for the management of recurrent bladder neck contractures. J Urol 2011;186:156-60. [Crossref] [PubMed]
- Lyon TD, Ayyash OM, Ferroni MC, et al. Bipolar Transurethral Incision of Bladder Neck Stenoses with Mitomycin C Injection. Adv Urol 2015;2015:758536. [Crossref] [PubMed]
- Redshaw JD, Broghammer JA, Smith TG, et al. Intralesional injection of mitomycin C at transurethral incision of bladder neck contracture may offer limited benefit: TURNS Study Group. J Urol 2015;193:587-92. [Crossref] [PubMed]
- Tavakkoli Tabassi K, Yarmohamadi A, Mohammadi S. Triamcinolone injection following internal urethrotomy for treatment of urethral stricture. Urol J 2011;8:132-6. [PubMed]
- Zhang K, Qi E, Zhang Y, et al. Efficacy and safety of local steroids for urethra strictures: a systematic review and meta-analysis. J Endourol 2014;28:962-8. [Crossref] [PubMed]
- Kumar S, Garg N, Singh SK, et al. Efficacy of Optical Internal Urethrotomy and Intralesional Injection of Vatsala-Santosh PGI Tri-Inject (Triamcinolone, Mitomycin C, and Hyaluronidase) in the Treatment of Anterior Urethral Stricture. Adv Urol 2014;2014:192710. [Crossref] [PubMed]
- Sangkum P, Yafi FA, Kim H, et al. Collagenase Clostridium histolyticum (Xiaflex) for the Treatment of Urethral Stricture Disease in a Rat Model of Urethral Fibrosis. Urology 2015;86:647. [Crossref] [PubMed]
- Elliott DS, Boone TB. Combined stent and artificial urinary sphincter for management of severe recurrent bladder neck contracture and stress incontinence after prostatectomy: a long-term evaluation. J Urol 2001;165:413-5. [Crossref] [PubMed]
- Hussain M, Greenwell TJ, Shah J, et al. Long-term results of a self-expanding wallstent in the treatment of urethral stricture. BJU Int 2004;94:1037-9. [Crossref] [PubMed]
- Magera JS Jr, Inman BA, Elliott DS. Outcome analysis of urethral wall stent insertion with artificial urinary sphincter placement for severe recurrent bladder neck contracture following radical prostatectomy. J Urol 2009;181:1236-41. [Crossref] [PubMed]
- De Vocht TF, van Venrooij GE, Boon TA. Self-expanding stent insertion for urethral strictures: a 10-year follow-up. BJU Int 2003;91:627-30. [Crossref] [PubMed]
- Adamakis I, Fragkiadis E, Katafigiotis I, et al. A two staged treatment procedure for the difficult to treat bladder neck contractures with concomitant incontinence. In the search of a solution to a complex problem. Arch Ital Urol Androl 2015;87:233-7. [Crossref] [PubMed]
- Yoon CY, Chae JY, Kim JW, et al. Transurethral resection of fibrotic scar tissue combined with temporary urethral stent placement for patients with in anterior urethral stricture. Int Braz J Urol 2014;40:576-7. [Crossref] [PubMed]
- Elliott SP, McAninch JW, Chi T, et al. Management of severe urethral complications of prostate cancer therapy. J Urol 2006;176:2508-13. [Crossref] [PubMed]
- Theodoros C, Katsifotis C, Stournaras P, et al. Abdomino-perineal repair of recurrent and complex bladder neck-prostatic urethra contractures. Eur Urol 2000;38:734-40. [Crossref] [PubMed]
- Wessells H, Morey AF, McAninch JW. Obliterative vesicourethral strictures following radical prostatectomy for prostate cancer: reconstructive armamentarium. J Urol 1998;160:1373-5. [Crossref] [PubMed]
- Hohenhorst J, Musch M, Pailliart A, et al. Robot-assisted laparoscopic YV-plasty in patients with refractory bladder bladder neck contracture. J Urol (Supp) 2016;193:e845. [Crossref]
- Anger JT, Raj GV, Delvecchio FC, et al. Anastomotic contracture and incontinence after radical prostatectomy: a graded approach to management. J Urol 2005;173:1143-6. [Crossref] [PubMed]
- Gousse AE, Tunuguntla HS, Leboeuf L. Two-stage management of severe postprostatectomy bladder neck contracture associated with stress incontinence. Urology 2005;65:316-9. [Crossref] [PubMed]
- Bates AS. Martin RM2 Terry TR. Complications following artificial urinary sphincter placement after radical prostatectomy and radiotherapy: a meta-analysis. BJU Int 2015;116:623-33. [Crossref] [PubMed]
- Cornu JN, Sèbe P, Ciofu C, et al. Mid-term evaluation of the transobturator male sling for post-prostatectomy incontinence: focus on prognostic factors. BJU Int 2011;108:236-40. [Crossref] [PubMed]
- Torrey R, Rajeshuni N, Ruel N, et al. Radiation history affects continence outcomes after advance transobturator sling placement in patients with post-prostatectomy incontinence. Urology 2013;82:713-7. [Crossref] [PubMed]
- Glass AS, McAninch JW, Zaid UB, et al. Urethroplasty after radiation therapy for prostate cancer. Urology 2012;79:1402-5. [Crossref] [PubMed]
- Meeks JJ, Brandes SB, Morey AF, et al. Urethroplasty for radiotherapy induced bulbomembranous strictures: a multi-institutional experience. J Urol 2011;185:1761-5. [Crossref] [PubMed]
- Hofer MD, Zhao LC, Morey AF, et al. Outcomes after urethroplasty for radiotherapy induced bulbomembranous urethral stricture disease. J Urol 2014;191:1307-12. [Crossref] [PubMed]
- Palmer DA, Buckley JC, Zinman LN, et al. Urethroplasty for high risk, long segment urethral strictures with ventral buccal mucosa graft and gracilis muscle flap. J Urol 2015;193:902-5. [Crossref] [PubMed]
- Ahyai SA, Schmid M, Kuhl M, et al. Outcomes of Ventral Onlay Buccal Mucosa Graft Urethroplasty in Patients after Radiotherapy. J Urol 2015;194:441-6. [Crossref] [PubMed]
- Fuchs JS, Hofer MD, Sheth KR, et al. Improving Outcomes of Bulbomembranous Urethroplasty for Radiation-induced Urethral Strictures in Post-Urolume Era. Urology 2017;99:240-245. [Crossref] [PubMed]
- Rourke K, Kinnaird A, Zorn J. Observations and outcomes of urethroplasty for bulbomembranous stenosis after radiation therapy for prostate cancer. World J Urol 2016;34:377-82. [Crossref] [PubMed]
- Riedmiller H, Kocot A. The devastated bladder outlet: treatment options. Curr Opin Urol 2015;25:352-6. [PubMed]
- Faris SF, Milam DF, Dmochowski RR, et al. Urinary diversions after radiation for prostate cancer: indications and treatment. Urology 2014;84:702-6. [Crossref] [PubMed]
- Hensle TW, Kirsch AJ, Kennedy WA, et al. Bladder neck closure in association with continent urinary diversion. J Urol 1995;154:883-5. [Crossref] [PubMed]
- Ullrich NF, Wessells H. A technique of bladder neck closure combining prostatectomy and intestinal interposition for unsalvageable urethral disease. J Urol 2002;167:634-6. [Crossref] [PubMed]


