Impact of the Ki-67 labeling index and p53 expression status on disease-free survival in pT1 urothelial carcinoma of the bladder
Introduction
In 2017, approximately 79,000 patients worldwide will be diagnosed with carcinoma of the urinary bladder (1). Approximately 80% of patients present with non-muscle-invasive bladder cancer (NMIBC) at diagnosis (2) and may be potentially cured by transurethral resection of bladder tumor (TURBT) without removing the organ (3). However, there is still a non-negligible subgroup of patients with NMIBC who harbor high-risk features (i.e., pTis, pT1, high grade/G3 tumors, or multifocal + recurrent + large tumors >3 cm) with a great propensity of disease recurrence and—more importantly—progression to muscle-invasive disease (3). Over the recent years, much effort has been put into identifying risk factors in those patients with high-risk NMIBC to optimize treatment recommendations with regard to adjuvant therapies (4,5). That said the role of protein biomarkers has been extensively explored in NMIBC (6-9). p53 as the result of the TP53 tumor suppressor gene (10) regulates cell cycle progression, senescence, and apoptosis following several stress signals (11). Consequently, molecular defects in human cancer cells have been linked to pathway irregularities with inactivated p53 leading to an increased half-life of the protein and prolonged detectability via immunohistochemistry (11,12). This concept has been adapted and validated for urothelial cancers as well (13-15). Likewise, Ki-67 constitutes an established protein biomarker encoded by the MKI67 gene (16) and is necessary for cellular proliferation. Against this backdrop, the proportion of Ki-67 positive tumor cells (i.e., the Ki-67 labeling index) is correlated with disease recurrence and progression in malignancies, and urothelial cancer in particular (14,15,17). However, not many studies focus on the expression patterns of aforementioned biomarkers in the subgroup of patients diagnosed with pT1 bladder cancer, and the available evidence is scarce and partially contradictory (13,18-22). The aim of our study was to mirror contemporary expression patterns of the p53 and Ki-67 biomarkers in a homogeneous Northern German cohort of patients with pT1 urothelial carcinoma of the bladder. We furthermore hypothesized that there is an association between marker positivity and disease recurrence.
Methods
Study population
We included patients undergoing endoscopic treatment due to cystoscopic evidence of a bladder tumor at four institutions in Northern Germany between 2009 and 2016. Only patients with pathologically confirmed pT1 urothelial carcinoma of the bladder according to the TNM staging system and those with complete data on Ki-67 and p53 expression status were considered for final analyses. The study was approved by the institutional review board and informed consent was taken from all the patients.
Covariates
Baseline patient characteristics were assessed by retrospective chart review and consisted of age at TURBT, gender, and age-adjusted Charlson comorbidity index (≤2, 3–5, and ≥6) (23). Tumor characteristics included the sequence of pT1 diagnosis (primary vs. progressive tumors), tumor focality at TURBT (unifocal vs. multifocal), concomitant carcinoma in situ, World Health Organization (WHO) 1973 grading, lymphovascular invasion at TURBT, and the administration of any adjuvant instillation therapy (Bacillus Calmette-Guérin or Mitomycin C).
p53 immunohistochemistry
The antibody DO1 (Oncogene; 1:3,600) was used for p53 protein detection after boiling the sections in an autoclave in citrate buffer (pH=7.8). The EnVision system (Dako) was used to visualize the immunostainings. Only intense p53 staining in almost all tumor nuclei was considered as positive and cytoplasmic staining was not considered as positive. Normal urothelial and non-epithelial cells (lymphocytes, stromal cells, and endothelial cells), used as negative controls, demonstrated no immunoreactivity.
Ki-67 immunohistochemistry
We used the Ki-67 antibody (Dako clone Mib-1; 1:400) to detect Ki-67 protein after heating the TURBT sections up to 98 °C for 20 minutes in citrate buffer (pH=9.0).
Ki-67 staining results were reported according to the proportion of Ki-67-positive bladder tumor cells in five percent increments, and only unequivocal nuclear staining, regardless of its intensity, was counted. Ki-67 labeling index was defined as positive when samples demonstrated >40% reactivity. As previously reported (24), we utilized the patient cohort reported in this study to assess the discriminative ability of Ki-67 as a categorical variable with 10% increments of cutoffs with regard to disease-free survival (DFS) (data not shown). Univariable Cox regression analyses revealed that the Ki-67 cutoff of 40% was the most superior discriminator for DFS.
Study endpoint
Our statistical endpoint was DFS, which was defined as the time interval between the date of TURBT with pT1 diagnosis to the date of any tumor recurrence or progression.
Statistical analyses
Our statistical analyses consisted of several steps.
First, we compared the distribution of patients with a negative and positive p53 and Ki-67 expression status according to baseline patient and tumor characteristics. Differences between the groups were assessed using Student’s t-test and one-way analysis of variance (ANOVA) for continuous variables (means and standard deviations; SDs), and Pearson’s χ2 test for categorical variables (frequencies and proportions), as appropriate.
Second, we calculated reverse Kaplan-Meier estimates for median follow-up time in censored patients (25) and plotted Kaplan-Meier curves (26,27) for DFS, comparing equality of survival curves between p53 negative vs. positive and Ki-67 negative vs. positive patients by the log-rank test (28).
Third, we performed univariable Cox regression analyses to calculate crude hazard ratios (HRs) for DFS for each of the aforementioned covariates, and for p53 and Ki-67 expression status. Covariates with significant univariable association with our endpoint were included into a multivariable Cox regression model to identify independent predictors of DFS. Given that p53 and Ki-67 expression status were our main covariates of interest, we included both into the multivariable model.
All statistical analyses were performed using Stata® (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP), and reported P values were two-sided and values <0.05 were considered statistically significant.
Results
Complete data on p53 expression was available in 102 patients, and information on the Ki-67 labeling index was available in 79 patients diagnosed with pT1 urothelial carcinoma of the bladder. Mean age of the p53 and Ki-67 cohort was 73.4 years (SD, 11.3 years) and 72.7 years (SD, 11.9 years), respectively. In both groups, about 80% of patients were men and had an age-adjusted Charlson comorbidity index of ≥3. In more than 90% of the patients in both groups, pT1 urothelial carcinoma was a primary bladder cancer diagnosis; the remaining 10% harbored recurrent tumors. In the p53 cohort, patients with a positive p53 expression status harbored concomitant carcinoma in situ more often (50.0% vs. 27.6%; P=0.032), and presented with WHO grade 3 more frequently (97.7% vs. 69.0%; P=0.001), as compared to their p53 negative counterparts. In the Ki-67 cohort, the mean Ki-67 labeling index was significantly higher in patients with WHO grade 3 vs. WHO grade 2 tumors (45.8 vs. 29.7; P=0.004), and thus, Ki-67 positivity—according to our definition—was found more often in patients with WHO grade 3 compared to WHO grade 2 tumors (93.3% vs. 71.4%; P=0.019). All baseline and tumor characteristics stratified according to p53 and Ki-67 expression status are reported in Table 1.
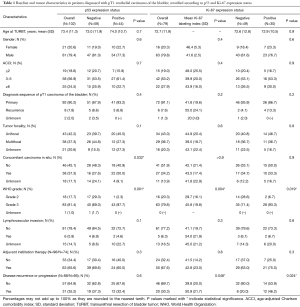
Full table
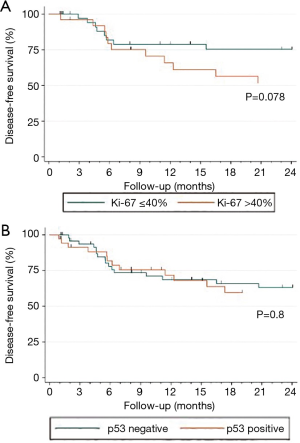
Of 91 patients with available information on follow-up, 31 (34.1%) suffered from disease recurrence. Patients with a follow-up <1 month were subsequently excluded to mitigate potential bias due to short follow-up intervals, yielding a final study population of 88 patients for survival analyses. Reverse Kaplan-Meier estimate of median follow-up was 51.0 months [95% confidence interval (CI), 24 to 56 months]. DFS curves stratified according to p53 and Ki-67 expression status are presented in Figure 1A and 1B, respectively. Kaplan-Meier curves in Ki-67 positive vs. Ki-67 negative patients were slightly separated, but were not significantly different (log-rank test: P=0.078). When comparing p53 positive vs. p53 negative patients, there was no difference in DFS (log-rank test: P=0.8).
In univariable Cox regression analyses, only lymphovascular invasion at TURBT was associated with shorter DFS (HR, 3.42; 95% CI, 1.18–9.89; P=0.023), and neither Ki-67 (P=0.8) nor p53 (P=0.086) expression status were associated with our endpoint. However, in multivariable analyses, adjusting for lymphovascular invasion and p53 expression status, Ki-67 positivity was significantly associated with adverse DFS (HR, 2.66; 95% CI, 1.02–6.95; P=0.046). Uni- and multivariable Cox regression analyses are reported in Table 2.
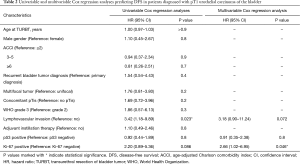
Full table
Discussion
In an era of tailored treatment and personalized risk-adapted therapeutic recommendations, many investigators have focused on identifying potential biomarkers to improve risk stratification in patients at highest risk of disease recurrence and progression. Indeed, patients with high-risk NMIBC according to the European Association of Urology (EAU) risk groups (3) warrant continuous and rigorous follow-up. Evaluating new prognostic tools on a microscopic and molecular level in TURBT specimens contains great potential of adding predictive value to already established risk factors for adverse oncological outcomes, and may ultimately guide adjuvant treatment decisions such as the administration of Bacillus Calmette-Guérin instillation therapy or early cystectomy. For example, current EAU guidelines advocate the use of pT1 substaging (3), although an optimal system remains to be defined (29,30). On a molecular level on the other hand, tumor suppressor gene TP53 product p53 and cell proliferation indicator Ki-67 levels in tumor cells are known to have significant impact on bladder cancer outcomes (18,31). However, to date there are no recommendations regarding routine use of protein biomarkers for risk stratification in NMIBC (3). To report the expression, detection, and clinical value of those biomarkers in a homogeneous cohort of patients, we evaluated immunohistochemistry staining by central pathological review of tumor specimen in patients with primary diagnosis of pT1 bladder cancer and depicted potential associations of protein overexpression and DFS. Several of our findings are noteworthy.
First, we found that patients with p53 or Ki-67 positivity presented with higher WHO grade at diagnosis and concomitant carcinoma in situ was found more often in patients with p53 positive tumors. Numerous previous studies have shown that p53 and Ki-67 may serve as a surrogate for high-grade NMIBC (10,18,20,22,32-34). Ki-67 is directly associated to cell proliferation (16) and a mutated TP53 gene results in higher levels of p53 protein expression due to a prolonged half-life of the protein (12). Thus, intuitively it seems reasonable that in tumors with high levels of a proliferation trigger and an overexpressed dysfunctional tumor suppressor, pathological parameters such as grading are worse compared to a tumor cell with moderate or no molecular alterations.
Second, in univariable Kaplan-Meier analyses, there was no difference in DFS when patients were stratified by biomarker positivity. However, after adjusting in multivariable analyses, Ki-67 positivity was significantly associated with shorter DFS. In general, evidence of the predictive value of solitary markers such as p53 and Ki-67 are contradictory, and most studies pool patients with different NMIBC stages rather than reporting outcomes for a specific subgroup (14,17,35-37). However, there are a number of studies explicitly focusing on pT1 bladder cancer, which corroborate our findings (19,20). For example, a study group from the Memorial Sloan-Kettering Cancer Center prospectively evaluated 89 patients with primary pT1 diagnosis and found no significant association between p53 overexpression and progression or survival (20). Similarly, a French study group found no independent predictive value of p53 regarding progression in 94 pT1 patients (19). Conversely, in a recent meta-analysis of studies evaluating the prognostic value of p53 in 712 pT1 bladder cancer patients, the authors suggest an association of p53 overexpression and progression [pooled risk ratio (RR), 2.32; 95% CI, 1.59–3.38; P<0.0001] (13).
The literature on the predictive value of Ki-67 expression in pT1 bladder cancer is contradictory as well. A Spanish study group evaluated tumor specimen by immunohistochemical staining in 175 patients with a pT1 diagnosis and found no prognostic value of Ki-67 when analyzing cancer-specific survival as an endpoint (22). Similarly, another Spanish group found an association of Ki-67 positivity and disease-free, progression-free, and overall survival in 51 patients with pT1 G3 bladder cancer in univariable analyses, which, however, did not hold true in multivariable models (21). Interestingly, in a recent systematic review and meta-analysis, the authors found an association of high Ki-67 expression and shorter recurrence-free survival (pooled HR, 1.45; 95% CI, 1.09–1.93; P=0.01) when summarizing six studies including 927 patients, which performed pT1 subgroup analyses (18). Similar trends were found for progression-free survival (pooled HR, 1.78; 95% CI, 1.22–2.60; P=0.003) and cancer-specific survival (pooled HR, 2.86; 95% CI, 1.16–7.02; P=0.02) when summarizing five and three studies including 799 and 695 patients, respectively (18).
Two things have to be considered when interpreting findings from aforementioned meta-analyses and the available primary data. First, almost all studies suffer from significant heterogeneity regarding the populations included. This is of particular relevance regarding the cutoff values used to define p53 or Ki-67 positivity. Given that there is no internationally approved cutoff for biomarker positivity, the presented findings have to be inferred with greatest caution, as patients were pooled without accounting for the different thresholds. In addition, there is no standardization regarding the confounding variables, which were adjusted for when presenting independent predictive value of p53 and Ki-67 using multivariable models. The most likely reason for this is a low number of samples and events when performing multivariable analyses, which may lead to over-fitting otherwise. Second, there has been a shift towards the implementation of combined biomarker panels and molecular subtyping in the NMIBC setting. Considering molecular relationships of different mRNA or protein biomarkers, this is a logical consequence in order to account for several molecular interactions regarding cell proliferation and tumor suppression. For example, Shariat et al. showed the predictive benefit of a combination of altered biomarkers (p53, p27, and Ki-67) over solitary biomarker positivity in 80 patients with pT1 bladder cancer (38). In another multicenter study, van Rhijn et al. validated the European Organization for Research and Treatment of Cancer (EORTC) risk scores as adopted in the EAU guidelines in a cohort of 230 patients with primary NMIBC and determined its relation to molecular grade based on fibroblast growth factor receptor 3 (FGFR3) gene mutation status and Ki-67 expression (8). Predictive accuracy for disease progression was increased from 74.9% to 81.7% when including molecular grade (P<0.001). Most recent studies have finally shown the value of distinguishing molecular subtypes based on different biomarker expression patterns to improve risk stratification and provide information on tumor progression. Sjodahl et al. suggested immunohistochemistry-based subtypes, defining urobasal, genomically unstable, and squamous-cell-carcinoma-like urothelial tumors (39). This subtype classification was validated in 167 patients with pT1 stage and rapidly progressing tumors were strongly associated with genomically unstable or squamous-cell-carcinoma-like tumors (40). Another approach is the molecular subtyping of bladder cancer according to distinct molecular classes resembling features of luminal and basal breast cancer subtypes (41), which was validated in 255 patients with pT1 disease (6). In short, future research will be highly dependent on evaluating different biomarker panels, microscopic, and molecular subtypes rather than focusing on singular protein biomarkers. However, in concordance with the available literature, our study suggests a certain role of Ki-67 within those potential biomarker panels, whereas the role of p53 remains unclear.
The presented findings have to be cautiously interpreted within certain limitations of our observational study design. First, given the retrospective nature of the data used, remaining selection bias is likely, as only patients with available information on p53 and Ki-67 expression from a pool of individuals with pT1 urothelial carcinoma were selected for analyses. Further, tumor size was available in a minority of our cohort, which made adequate risk adjustment for this variable impossible. Given that tumor size is an established risk factor of disease recurrence in NMIBC, this may have introduced a certain bias into our analyses. Second, no officially approved threshold exists regarding the Ki-67 labeling index to define Ki-67 positivity in urinary bladder cancer (18). We attempted to evaluate the threshold in our cohort to provide highest precision in predicting DFS using a method, which has been previously described (24). However, residual inaccuracies due to the relatively small sample size cannot be ruled out. Moreover, given that number of events was quite low, our multivariable regression model was prone to over-fitting, which we attempted to mitigate by purposefully selecting covariates for this model, and including only those showing a significant univariable association with our endpoint as well as our main variables of interest. Third, the purpose of this study was not to frame a robust statement on whether p53 and Ki-67 expression analyses should or should not be routinely performed. We rather sought to mirror the expression patterns in a contemporary series of patients, and to delineate potential implications for subsequent risk stratification and treatment in those patients at highest risk of disease recurrence and progression. In addition, our findings may be of importance regarding future study design in pT1 bladder cancer.
In conclusion, we found an association of a Ki-67 labeling index >40 at TURBT and shorter DFS in a contemporary sample of patients diagnosed with pT1 urothelial carcinoma of the bladder. Conversely, the predictive benefit of p53 expression in those patients seemed to be marginal in our patient cohort. Given the non-negligible risk of disease recurrence and progression in pT1 bladder cancer patients, there is further need of identifying additional risk factors and biomarker panels, which may improve risk stratification to guide adjuvant therapies, such as intravesical instillation therapy or early cystectomy. Specifically in the case of Ki-67, large prospectively planned studies are warranted to adequately evaluate optimal thresholds while adjusting for all relevant cofactors with regard to DFS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethnical Statement: The study was approved by the institutional review board and informed consent was taken from all the patients.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Nielsen ME, Smith AB, Meyer AM, et al. Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006. Cancer 2014;120:86-95. [Crossref] [PubMed]
- Babjuk M, Bohle A, Burger M, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Cambier S, Sylvester RJ, Collette L, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guerin. Eur Urol 2016;69:60-9. [Crossref] [PubMed]
- Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466-5; discussion 475-7.
- Breyer J, Wirtz RM, Otto W, et al. Predictive value of molecular subtyping in NMIBC by RT-qPCR of ERBB2, ESR1, PGR and MKI67 from formalin-fixed TUR biopsies. Oncotarget 2017;8:67684-95. [PubMed]
- van Rhijn BW, Vis AN, van der Kwast TH, et al. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J Clin Oncol 2003;21:1912-21. [Crossref] [PubMed]
- van Rhijn BW, Zuiverloon TC, Vis AN, et al. Molecular grade (FGFR3/MIB-1) and EORTC risk scores are predictive in primary non-muscle-invasive bladder cancer. Eur Urol 2010;58:433-41. [Crossref] [PubMed]
- Rink M, Cha EK, Green D, et al. Biomolecular predictors of urothelial cancer behavior and treatment outcomes. Curr Urol Rep 2012;13:122-35. [Crossref] [PubMed]
- Esrig D, Spruck CH 3rd, Nichols PW, et al. p53 nuclear protein accumulation correlates with mutations in the p53 gene, tumor grade, and stage in bladder cancer. Am J Pathol 1993;143:1389-97. [PubMed]
- Brown CJ, Lain S, Verma CS, et al. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer 2009;9:862-73. [Crossref] [PubMed]
- Finlay CA, Hinds PW, Tan TH, et al. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol 1988;8:531-9. [Crossref] [PubMed]
- Du J, Wang SH, Yang Q, et al. p53 status correlates with the risk of progression in stage T1 bladder cancer: a meta-analysis. World J Surg Oncol 2016;14:137. [Crossref] [PubMed]
- Wang L, Feng C, Ding G, et al. Ki67 and TP53 expressions predict recurrence of non-muscle-invasive bladder cancer. Tumour Biol 2014;35:2989-95. [Crossref] [PubMed]
- Krause FS, Feil G, Bichler KH. Immunohistochemical examinations (Ki67, p53, nm23) and DNA cytophotometry in bladder cancer. Anticancer Res 2000;20:5023-8. [PubMed]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000;182:311-22. [Crossref] [PubMed]
- Ding W, Gou Y, Sun C, et al. Ki-67 is an independent indicator in non-muscle invasive bladder cancer (NMIBC); combination of EORTC risk scores and Ki-67 expression could improve the risk stratification of NMIBC. Urol Oncol 2014;32:42.e13-9. [Crossref] [PubMed]
- Tian Y, Ma Z, Chen Z, et al. Clinicopathological and Prognostic Value of Ki-67 Expression in Bladder Cancer: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0158891. [Crossref] [PubMed]
- Bernardini S, Billerey C, Martin M, et al. The predictive value of muscularis mucosae invasion and p53 over expression on progression of stage T1 bladder carcinoma. J Urol 2001;165:42-6; discussion 46. [Crossref] [PubMed]
- Dalbagni G, Parekh DJ, Ben-Porat L, et al. Prospective evaluation of p53 as a prognostic marker in T1 transitional cell carcinoma of the bladder. BJU Int 2007;99:281-5. [Crossref] [PubMed]
- Lopez-Beltran A, Luque RJ, Alvarez-Kindelan J, et al. Prognostic factors in stage T1 grade 3 bladder cancer survival: the role of G1-S modulators (p53, p21Waf1, p27kip1, Cyclin D1, and Cyclin D3) and proliferation index (ki67-MIB1). Eur Urol 2004;45:606-12. [Crossref] [PubMed]
- Rodríguez-Alonso A, Pita-Fernandez S, Gonzalez-Carrero J, et al. p53 and ki67 expression as prognostic factors for cancer-related survival in stage T1 transitional cell bladder carcinoma. Eur Urol 2002;41:182-8; discussion 88-9. [Crossref] [PubMed]
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. [Crossref] [PubMed]
- Margulis V, Lotan Y, Karakiewicz PI, et al. Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. J Natl Cancer Inst 2009;101:114-9. [Crossref] [PubMed]
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343-6. [Crossref] [PubMed]
- Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc 1958;53:457-81. [Crossref]
- Rink M, Kluth LA, Shariat SF, et al. Kaplan-Meier analysis in urological practice. Urologe A 2013;52:838-41. [Crossref] [PubMed]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966;50:163-70. [PubMed]
- van Rhijn BW, van der Kwast TH, Alkhateeb SS, et al. A new and highly prognostic system to discern T1 bladder cancer substage. Eur Urol 2012;61:378-84. [Crossref] [PubMed]
- Martin-Doyle W, Leow JJ, Orsola A, et al. Improving selection criteria for early cystectomy in high-grade T1 bladder cancer: a meta-analysis of 15,215 patients. J Clin Oncol 2015;33:643-50. [Crossref] [PubMed]
- George B, Datar RH, Wu L, et al. p53 gene and protein status: the role of p53 alterations in predicting outcome in patients with bladder cancer. J Clin Oncol 2007;25:5352-8. [Crossref] [PubMed]
- Ben Abdelkrim S, Rammeh S, Ziadi S, et al. Expression of topoisomerase II alpha, ki67, and p53 in primary non-muscle-invasive urothelial bladder carcinoma. J Immunoassay Immunochem 2014;35:358-67. [Crossref] [PubMed]
- Goebell PJ, Groshen SG, Schmitz-Drager BJ, et al. p53 immunohistochemistry in bladder cancer--a new approach to an old question. Urol Oncol 2010;28:377-88. [Crossref] [PubMed]
- van Rhijn BW, Liu L, Vis AN, et al. Prognostic value of molecular markers, sub-stage and European Organisation for the Research and Treatment of Cancer risk scores in primary T1 bladder cancer. BJU Int 2012;110:1169-76. [Crossref] [PubMed]
- Gil P, Allepuz C, Blas M, et al. Significance of protein p53 overexpression in the clinical course of high-risk superficial bladder cancer. Urol Int 2003;70:172-7. [Crossref] [PubMed]
- Santos L, Amaro T, Costa C, et al. Ki-67 index enhances the prognostic accuracy of the urothelial superficial bladder carcinoma risk group classification. Int J Cancer 2003;105:267-72. [Crossref] [PubMed]
- Gontero P, Casetta G, Zitella A, et al. Evaluation of P53 protein overexpression, Ki67 proliferative activity and mitotic index as markers of tumour recurrence in superficial transitional cell carcinoma of the bladder. Eur Urol 2000;38:287-96. [Crossref] [PubMed]
- Shariat SF, Bolenz C, Godoy G, et al. Predictive value of combined immunohistochemical markers in patients with pT1 urothelial carcinoma at radical cystectomy. J Urol 2009;182:78-84; discussion 84. [Crossref] [PubMed]
- Sjödahl G, Lauss M, Lovgren K, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res 2012;18:3377-86. [Crossref] [PubMed]
- Patschan O, Sjodahl G, Chebil G, et al. A Molecular Pathologic Framework for Risk Stratification of Stage T1 Urothelial Carcinoma. Eur Urol 2015;68:824-32; discussion 835-6. [Crossref] [PubMed]
- Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A 2014;111:3110-5. [Crossref] [PubMed]


