Magnetic resonance imaging in active surveillance—a modern approach
Introduction
Prostate cancer is the most common malignancy in men, with over 1,600,000 cases and 366,000 deaths annually worldwide (1). Population screening using prostate specific antigen (PSA) has led to a decrease in cancer-related mortality but also to an increased detection of patients diagnosed with low-risk biopsy proven prostate cancer (2). The impact of PSA screening on overall mortality remains unclear, and seems to differ depending on the population and strategy used (3). The choice of treatment for a man with localised prostate cancer is determined by the risk of the cancer progressing, and patient preferences. Patients are commonly asked to balance the avoidance of side effects on active surveillance with a slight increase in the need for more intensive treatment, when choosing an initial management strategy for a new diagnosis of lower risk prostate cancer.
Risk stratification is based on different parameters such as clinical stage, PSA, Gleason score and an approximation of cancer volume (indicated by the number of positive cores and the maximum extent of cancer within a positive core at biopsy) (4). In recent years, active surveillance has been increasingly adopted for conservative management of low and sometimes intermediate risk prostate cancer to avoid treatment until there is evidence of higher risk disease (5). Published active surveillance protocols rely on PSA, digital rectal examination and biopsy results, and they differ by institution (5,6). The majority of them consider only low risk Gleason 3+3 disease, but some centres also allow men with lower intermediate risk features such as Gleason 3+4 disease (6,7).
There is evidence that multiparametric magnetic resonance imaging (mpMRI) in the setting of active surveillance for prostate cancer is being increasingly used (8).
It is well known that the negative predictive value of high quality mpMRI for the detection of clinically significant cancer is very high (8-10). Studies have also shown that the presence of a visible lesion on mpMRI in men on active surveillance for prostate cancer increases the likelihood of higher risk disease at subsequent biopsy (11,12).
In 2012 a systematic review of mpMRI targeted biopsies compared to standard biopsies in the diagnostic setting showed that MRI-targeted biopsies are at least as accurate at detecting clinically significant disease, and can do so with greater efficiency (fewer cores taken in fewer men) (13). MRI-targeted biopsy was defined as any technique which used the information gained on the presence and location of a suspicious lesion on mpMRI, at the time of the biopsy.
The biopsy itself can be carried out using one of three methods, as set out by the START (Standards of Reporting for MRI-targeted Biopsy Studies) collaborative group (14):
- In-bore biopsy, where diagnostic MRI images are fused with lower strength interventional MR images, and the biopsy is carried out in the MRI scanner;
- Visual registration, where the information on the MRI suspicious lesion is conveyed to the biopsy operator by means of a diagram or snapshotted image in the MRI data, and the biopsy is carried out using transrectal ultrasound;
- Software assisted registration, where the MRI lesion is fused with or overlaid on the real-time ultrasound image used at the time of biopsy.
More recently, Wegelin and colleagues assessed which of the different ways of using the mpMRI information at the time of biopsy was more accurate (15). In their review, they concluded that MRI-guided biopsies (all techniques combined) have higher detection rates of clinically significant prostate cancer compared to transrectal ultrasound guided biopsies on a per patient basis, with a lower rate of detection of insignificant disease. For the detection of clinically significant prostate cancer there was no difference in efficacy between the different MRI-guided techniques. They also noted that MRI-guided biopsies miss 10% of significant cancers, compared to 21% missed using a standard biopsy approach alone.
De Visschere and colleagues (16) have recently shown that men with normal findings on mpMRI (overall Prostate Imaging Reporting and Data System-PI-RADS- ≤2) are at very low risk of having a clinically significant prostate cancer, with negative predictive values ranging between 63% and 91% for prostate cancer of any grade, and from 92% to 100% for clinically significant prostate cancer (depending on the definition used) in low-risk men (PSA <10 ng/mL, normal digital rectal examination, no family history). This could have implications for a re-biopsy strategy in men with low risk disease on active surveillance, who might be able to avoid or defer repeat biopsy if they are deemed to have no visible cancer on mpMRI.
It is also established that MRI-targeted biopsies are able to better characterise prostate cancer with regards to Gleason score and cancer burden (17,18). In some centres, mpMRI and MRI-guided biopsies are used as an additional testing method to look for evidence that would prompt a change to active management of prostate cancer (8). For men with low risk cancer on initial standard biopsy who opt for active surveillance, they should be counselled to have additional MRI-targeted biopsies if the mpMRI shows a suspicious lesion which has not been demonstrated on biopsy. The risk of upgrading in men on active surveillance with non-visible cancer on mpMRI has been shown by one group to be low (8.3%) vs. 41% in men with visible tumour on mpMRI (19).
Another paper assessing the concordance of biopsy and mpMRI in men on active surveillance concluded that those men who had a concordant mpMRI and biopsy could safely avoid confirmatory biopsy, whereas those with an MRI lesion >1 cm should be counselled to have an MRI-targeted biopsy (20).
In the last few years the scientific community has shown an increasing interest in the use of mpMRI in active surveillance (21-23). There is discussion about whether all of the potential mpMRI sequences should be used at all time points in men on active surveillance. Recent papers include systematic reviews, editorials, commentaries and original studies. In this report, we collate and discuss those papers—classified as original articles—that investigate the role of the different mpMRI sequences in men on active surveillance for prostate cancer.
Multiparametric MRI in active surveillance protocols
A detailed explanation of mpMRI of the prostate is beyond the purpose of this paper, but some technical concepts must be discussed in order to fully understand the studies on active surveillance that will be presented.
MpMRI refers to the use of different anatomical and functional imaging parameters, each of which investigates a specific aspect of the prostate gland.
T2-weighted imaging (T2-WI): this is the most useful technique to study the anatomy of the gland. The peripheral zone appears bright due to the high presence of glandular tissue, while the transitional zone shows a heterogeneous appearance with the presence of multiple stromal nodules made of compact muscle fibre bundles. Tumours on T2-WI show low signal intensity (i.e., darker than surrounding tissues) (24).
Diffusion-weighted magnetic resonance imaging (DW-MRI): this sequence assesses the free movement (i.e., the diffusion) of water molecules within tissues. In prostate cancer, this results in a higher signal intensity (i.e., brighter than surrounding tissues) on long b-value sequences and a lower signal (dark areas) on the reconstructed apparent diffusion coefficient (ADC) map (25,26). Lower ADC values have been shown to correlate with higher Gleason grade tumours on active surveillance (27).
Dynamic contrast enhanced (DCE) sequences: this refers to the intravenous administration of a specific contrast agent, most commonly gadolinium. Prostate cancer usually shows early wash in and wash out, due to its leaky and disorganised vasculature (28).
Magnetic resonance spectroscopy: this technique analyses the different metabolites in normal and pathological tissues. Cancerous cells contain more choline due to increased cell turnover which leads to an increased choline+creatine/citrate (29).
Figure 1 and Figure 2 show the appearance of prostate cancer in the peripheral and transitional zone on T2-WI, DWI and DCE sequences, respectively.
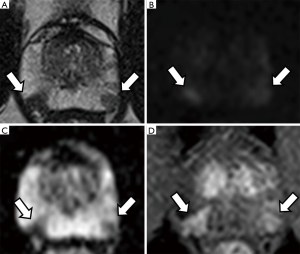
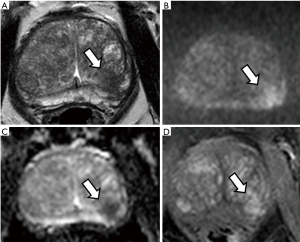
Two other important aspects of mpMRI of the prostate should be mentioned:
Coils: mpMRI of the prostate is performed using a pelvic phased-array coil, with some institutions using an additional endorectal coil (30). However, a recent study has shown that the use of an endorectal coil can be omitted in a prostate cancer detection setting (31).
Scoring system: the likelihood of clinically significant prostate cancer on mpMRI is commonly reported using a 1-5 likelihood scale. The PI-RADS scale is an internationally agreed set of criteria to determine the score (32-34) whereas the Likert scale is less prescriptive but allows an individual radiologist to give an overall impression of the likelihood of clinically significant disease (35).
The ability of mpMRI to rule out clinically-significant prostate cancer prior to diagnostic biopsy has been shown in the PROMIS study, a prospective, multi-centre trial conducted in the United Kingdom (36). The study was conducted on 576 biopsy-naïve men, who underwent mpMRI followed by both transrectal ultrasound and transperineal template prostate mapping biopsy. This latter was used as reference standard. Clinically significant cancer was defined as Gleason score ≥4+3, or a maximum cancer core length 6 mm or more in any location. The negative predictive value in detecting clinically significant cancer was 89% for mpMRI and 74% for transrectal ultrasound biopsy (P<0.0001). Additionally, the results from this study supports the idea that mpMRI, used as a triage test before first prostate biopsy, could reduce unnecessary biopsies by a quarter. The authors suggest that mpMRI could be recommended to all men with an elevated serum PSA before biopsy, reducing the diagnosis of clinically insignificant disease and improving the detection of clinically significant cancers.
The negative predictive value of mpMRI demonstrated here has relevance for the application of mpMRI in active surveillance for prostate cancer. The UK National Institute for Health and Care Excellence (NICE) guidelines recommend the use of mpMRI at the start of active surveillance, and that it can be used either in addition to or instead of biopsy for repeat assessment during follow up (37).
In a recent study by Tran et al. 207 men on active surveillance underwent MRI-ultrasound fusion biopsy, and 14% had pathological upgrading in the targeted cores that was not detected by systematic sampling (38).
There is discussion about whether it might be appropriate to instigate active treatment based on mpMRI alone in men with biopsy proven prostate cancer on active surveillance, or whether a targeted biopsy must always be done prior to definitive treatment. Whilst biopsy to confirm any mpMRI findings is usually recommended, there are patients who could have opted for active treatment rather than active surveillance who will wish to avoid further biopsy prior to treatment (17).
Evidence acquisition
In this paper, we have assessed those studies which have analysed the role of the different mpMRI sequences (T2, DWI, DCE and spectroscopy) in men on active surveillance for prostate cancer. We searched MEDLINE/PubMed for manuscripts published from the inception of PubMed in 1971 to 1st December 2017. The search terms used were (prostate cancer or prostate adenocarcinoma or prostatic carcinoma or prostate carcinoma or prostatic adenocarcinoma) and (MRI or NMR or magnetic resonance imaging or mpMRI or multiparametric MRI) and active surveillance. If it was not clear from the abstract whether the paper might contain relevant data, the full paper was assessed.
Overall, 425 publications were found. Seventy-one papers were related to the research question. We then excluded those falling in one of the following groups: (I) review articles on active surveillance; (II) reports from consensus meetings, editorials, commentaries and case reports; (III) full text papers not in English. Twenty-five papers are included in the analysis. The literature search and study selection are displayed in Figure 3.
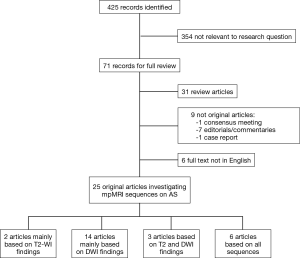
Evidence synthesis
Among the 25 reports which were assessed in full, two (8%) had their main results based on T2-WI, 14 (56%) on DWI, 3 (12%) on T2 and DWI, and 6 (24%) investigated more than two parameters of T2-WI, DWI, DCE and spectroscopy) (Figure 3).
We have collated the papers based on the sequences used.
T2-weighted imaging in active surveillance (Table 1)

Full table
Two studies from our own institution report interesting results based on calculations made on T2-WI (39,40). Both reports are from a randomised, placebo-controlled trial that evaluates the effects of 0.5 mg daily dutasteride for 6 months on prostate cancer. Men taking dutasteride are compared to those men on placebo. This is interesting in terms of the effect of dutasteride, but also for the use of repeat mpMRI in men on active surveillance, with the same set of sequences taken at baseline, 3 and 6 months in all men. The first paper (40) reported an average reduction in lesion volume of 36% on T2-WI in men on dutasteride. Conversely, in the placebo group there was an average increase in volume of 12% over the six-month period. The authors concluded that dutasteride was associated with a significant reduction in apparent prostate cancer volume on T2-weighted magnetic resonance imaging compared to placebo.
The other paper reported a retrospective review of the same cohort of men, looking at T2-relaxation time (a biomarker sensitive to tissue microenvironment) between baseline and 6-month mpMRI scans (39). T2 relaxation times and T2-WI were not significantly influenced by the exposure to dutasteride. It was concluded that dutasteride may not impair the ability to measure tumour volume on T2-WI in men on active surveillance for prostate cancer.
Diffusion weighted imaging in active surveillance (Table 2)
Full table
We found 14 studies whose main results are based on DWI findings, which are presented in Table 2. The majority of them are retrospective studies, but five studies were conducted prospectively (27,44,49,50,52). Five studies (27,41,45,48,49) included patients according to the Prostate Cancer Research International Active Surveillance (PRIAS) criteria (53), which is limited to Gleason 3+3 disease. However, four studies (25,50-52) enrolled also men with intermediate risk disease (Gleason 3+4). Five studies (27,43,50-52) used an endorectal coil in the mpMRI protocol, and some studies had whole gland pathology in men with biopsies suitable for active surveillance as a reference standard (41,45-48). One study (42) did not report the Gleason score prior to enrolment into active surveillance.
A closer look at Table 2 shows that the majority of the studies have been conducted on 3.0 T MRI scanners without an endorectal coil, and that 12 out of 14 (86%) report quantitative data from ADC calculations. As an example, our group at University College London has recently undertaken specific analyses on the effects of dutasteride on DWI (25). We have observed that absolute changes in ADC and lesion conspicuity on DWI are significantly different between men on active surveillance taking daily placebo or dutasteride for 6 months: (−0.03 vs. 0.08, P=0.033) and (0.11 vs. −0.16, P=0.012), respectively. The same has been observed for percentage changes in these parameters: (−2.27% vs. 8.56%, P=0.048) and (9.25% vs. −9.89%, P=0.013), respectively. Whilst dutasteride is not licenced for use in prostate cancer, it is not unusual for men on active surveillance to be taking a 5 alpha-reductase inhibitor for lower urinary tract symptoms, within the licence. With these men, it is worth remembering in clinical practice that the MRI conspicuity of prostate cancer may be reduced, and so having a lower threshold for biopsy or repeat biopsy in these men makes sense.
Three papers investigated the relationship between tumour volume and progression on active surveillance. Specifically, the results by Tamada et al. (42) suggest that changes in the lesion volumes on the ADC map by planimetry are associated with Gleason score on subsequent targeted biopsy. Lee and colleagues (48) analysed the whole gland pathological outcomes of men with pre-operative data meeting active surveillance requirements, stratified by maximal tumour diameter on the ADC map pre-operatively. They created two groups (group 1: normal mpMRI or suspicious tumour <1 cm; group 2: suspicious tumour >1 cm) and analysed whether different diameters resulted in a change in insignificant prostate cancer rates at radical prostatectomy. The rate of insignificant prostate cancer (defined as organ confined, Gleason 6 disease with tumour volume less than 0.5 cm3) was different between the two groups (48.7% vs. 24.7%; P=0.001, respectively). The rate of insignificant prostate cancer decreased as MRI defined tumour diameter increased over 1 cm. It is interesting to note that 4 men with apparent tumour on mpMRI of >1 cm diameter were deemed T0 at final pathology, although this is not specifically discussed in the text.
Giles and colleagues (50) observed that median lesion volume calculated on the ADC map by planimetry was significantly higher in men who were upgraded on repeat biopsy compared with those who were not (0.59 vs. 0.21 cm3, respectively; P=0.02).
Median tumour volume was also significantly different in those men who progressed to radical treatment compared with those who did not progress (0.68 vs. 0.22 cm3, respectively; P=0.02).
Two papers compared PI-RADS v. 2 scoring and ADC values in men with biopsies suitable for active surveillance who went on to have radical prostatectomy. Yim et al. (41) divided the patients into two groups based on the PI-RADS v. 2 score (≤3 vs. 4–5) and tumour ADC (using a threshold of 1.095×10−3 mm2/s). Insignificant cancer was defined on radical specimens as organ-confined disease with a tumour volume <0.5 cm3 and no Gleason score 4–5. PI-RADS v. 2 score ≤3 and ADC ≥1.095×10−3 mm2/s were independent predictors of insignificant cancer at multivariate analysis. They also observed a moderate negative correlation (Spearman rho=−0.653; P<0.001) between these two parameters, i.e., men with a low PI-RADS score were likely to have a higher ADC score, as might be expected as each parameter indicates a lower risk of clinically significant disease.
Nougaret and colleagues (43) reported that PI-RADS v. 2 was superior to ADC measurements for predicting prostate cancer upgrading. However, it should be noted that the reference standard of this study was confirmatory biopsy and not radical prostatectomy.
Jeong et al. (46) evaluated whether a 5-point Likert scale for the radiologist impression of the likelihood of clinically significant disease, based on the ADC map, was useful to identify men with unfavourable pathology within an active surveillance cohort. Unfavourable pathological features were defined as non–organ-confined disease or pathological Gleason score ≥4+3. The ADC image was scored according to a 1–5 scale, and the score was then qualitatively dichotomised into (≤3) vs. (>3). Radical prostatectomy was the reference standard. The rate of unfavourable pathological features was significantly different between lower (≤3) and higher (>3) grades on the ADC scale (3.5% vs. 28.1%, respectively; P<0.001). However, the authors used only two b values (0 and 1,000 s/mm2) and did not perform any quantitative analysis for ADC calculations, which can vary from one MRI scanner to another.
Looking at the studies done, it is clear that DWI shows great potential in identifying men with higher risk disease who have been classified as low risk disease suitable for active surveillance on standard biopsy. The potential advantage of using T2-weighted imaging and diffusion (sometimes referred to as bi-parametric MRI) is the shorter scan time (compared to the addition of contrast), and the fact that there does not need to be a doctor on site to cover for the potential for an allergic reaction to contrast.
Studies reporting T2-WI and DWI in active surveillance (Table 3)

Full table
Three studies report interesting results using combined data from T2-WI and DWI, and they are listed in Table 3. All three studies (54-56) have been conducted using an endorectal coil and with biopsy as the reference standard. Interestingly, none of them included DCE with gadolinium in the mpMRI protocols.
DeSouza and colleagues (56) compared tumour ADC values between men with clinically localised prostate cancer classified in two groups, as low risk (i.e., stageT1/T2a, Gleason score ≤6, PSA <10 ng/mL) vs intermediate/high risk of progression. They contoured tumour volume on T2-WI by planimetry, and then transferred the contours on the isotropic ADC maps to get mean ADC values. The authors concluded that tumour volume on T2-WI (P=0.002) and ADCslow (P=0.005) were both significant predictors of higher risk disease.
Flavell et al. (55) conducted a study where T2, DWI and spectroscopy were included in the mpMRI protocol. T2-WI was assessed according to a 5-point scale, while DWI was classified according to a binary scale (normal vs. abnormal). An abnormal DWI was characterised by the presence of a region of low signal intensity, with volume greater than 0.03 cc, with ADC values lower than 1,100×10−6 mm2/s for the echo-planar imaging, and 900×10−6 mm2/s for the fast spin echo imaging. MR spectroscopic imaging was scored in a binary manner (positive vs. negative). Gleason score upgrade (i.e., any Gleason pattern 4 or 5) at subsequent biopsies was reported. At multivariate analysis, when considering all imaging sequences together, only a T2-WI score of 4 or 5 or abnormal DWI were independent predictors of biopsy upgrade (T2-WI: hazard ratio=2.46; P=0.003 and DWI: hazard ratio=2.76; P=0.03).
Whilst many studies of mpMRI simply assess the use of single mpMRI at baseline or when mpMRI is introduced at an institution, there is great interest in the use of mpMRI to detect change over time. Morgan and colleagues (54) investigated the change in tumour volume over time in 151 men (median interval was 1.9 years) to determine whether baseline ADC and ADC changes were predictive of tumour growth. All patients underwent biopsy a median of 1.7 years before the baseline mpMRI. Re-biopsy data were available after a greater than 24-month interval only for 47 patients. They contoured tumour volume on T2-WI by planimetry, and then transferred the contours on the ADC maps. ADC was measured on the slice with the largest lesion. The authors concluded that change in T2-WI volume correlates with a change in ADC; ADC may therefore be used to identify men with clinically significant growth, suggesting a 5.8% reduction in ADC as a possible threshold (specificity: 77%; sensitivity:54.9%) for indicating volume progression. However, it should be kept in mind that at present there are no clear recommendations on how the ADC should be calculated from the map (e.g., drawing a single region of interest or by planimetry). Also, ADC absolute values can vary from one MRI system to the other, and they are dependent on the number of b values acquired during the scan.
Use of multiple (at least 3 sequences) in men on active surveillance (Table 4)
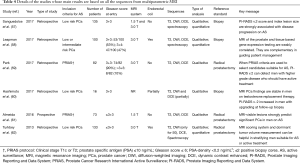
Full table
Six studies have assessed multiple MR parameters in men on active surveillance, and they are listed in Table 4. Two studies (59,61) included men according to PRIAS criteria. Almeida et al. (61) used DWI and DCE to score the lesions in 73 men according to PI-RADS v.2 (33) guidelines, but they also conducted axial, coronal and sagittal T2-WI acquisitions that helped in the localisation of the tumour. PI-RADS score was correlated with pathological data to evaluate the prognostic role of mpMRI from men with biopsies suitable for active surveillance who chose radical prostatectomy. Upgrading, upstaging and unfavourable disease were reported. Upgrading was defined by whole gland pathological Gleason score ≥7, and upstaging when pathological staging ≥pT3a. Unfavourable disease was defined when upgrading and/or upstaging were seen, and PI-RADS score was >3. They concluded that MRI-visible lesions strongly predict significant prostate cancer in men on active surveillance.
Park et al. (59) retrospectively evaluated the role of PI-RADS v. 2 in assessing men with prostate cancer who met PRIAS criteria. From an initial cohort of men (n=1,122) receiving radical prostatectomy for prostate cancer, they identified 456 men who met the PRIAS criteria (i.e., clinical stage <T3, PSA ≤10 ng/mL, PSA density ≤0.2 ng/mL2, Gleason score ≤6), with no previous history of hormone or radiation treatment or transurethral resection of the prostate, and with an adequate number of biopsy cores and mpMRI scan quality. Significant prostate cancer at radical prostatectomy was defined as Gleason >6 and pathological cancer volume ≥0.5 cc. Among these 456 patients, 374 (82%) had significant cancer on prostatectomy specimens. The remaining 82 (18%) had insignificant cancer suitable for active surveillance. Two radiologists independently reported all scans according to PI-RADS v. 2 guidelines, and those scans with an index lesion scored <4 were considered suitable for active surveillance.
They concluded that PI-RADS v. 2 can detect many significant cancers that are misdiagnosed as insignificant cancer with PRIAS, and suggested that candidates suitable for active surveillance should be detected with PRIAS alone, and then their MR images should be assessed using PI-RADS v. 2 to exclude significant cancers (i.e., false-positive candidates for active surveillance).
In a recent study by Sanguedolce and colleagues (57) PI-RADS v. 2 and Likert scores were assessed on baseline mpMRI of 135 men on active surveillance. Other parameters, including index lesion size, were also assessed, and Kaplan-Meier survival curves were calculated with respect to patient withdrawal from active surveillance and failure-free survival. At multivariate analysis, the variables significantly associated with failure-free survival were the index lesion size (dichotomised as ≤10 and >10 mm) and PI-RADS v. 2 score (≤3 vs. 4–5).
Turkbey et al. (62) have also provided compelling evidence that mpMRI can add important information to clinic-pathologic scoring systems. Two experienced radiologists evaluated T2-WI, ADC maps, DCE and spectroscopy images in consensus, in 133 men before radical prostatectomy and assigned an imaging score to each lesion. For segmenting the tumours, T2-WI, ADC maps and DCE images were used to determine tumour boundaries in combination, although the final calculations were made on T2-WI. The authors concluded that MRI scoring system and dominant tumour volume measurements could be helpful in stratifying men suitable for active surveillance or active treatment.
One study (58) has compared mpMRI findings with a genomic prostate score based on a biochemical 17-gene expression signature. A 5-point scale of increasing suspicion of malignancy was used, and ADC was calculated from the lesions. As far as the genomic prostate score is concerned, no significant differences were seen for Gleason 3+3 (P=0.179), but significant results were observed with Gleason 3+4 (P=0.01). Mean ADC was weakly correlated with the genomic prostate score.
Hashimoto et al. (60) analysed the changes in prostate mpMRI features after testosterone replacement in 12 men on active surveillance and evaluated the ability of mpMRI to detect disease progression in this cohort. From the initial population (n=16), three men discontinued the therapy for cancer progression (n=1), increased haematocrit (n=1) and explicit request (n=1). The serum testosterone levels in the remaining twelve patients reached reference range at 6 months after the replacement therapy initiation. The authors of the paper reported that mpMRI findings were stable in those patients without progression at biopsy, while PI-RADS v. 2 score increased in two patients who were also upgraded (Gleason 3+4) on follow-up biopsy.
Conclusions
Active surveillance is a promising management strategy for low and intermediate risk prostate cancer. MpMRI has been shown to have a bearing both in the diagnosis and follow-up of this disease, including active surveillance. This technique can provide important, additional information on the identification (e.g., PI-RADS v. 2 or Likert scoring system) and characterisation (mpMRI targeted biopsy) of prostate cancer. At this regard, it is known that systematic transrectal ultrasound biopsy can miss a substantial proportion of significant prostate cancer and there is growing evidence that using mpMRI to target biopsy improves the accuracy of classification, and may overcome the sampling errors that might occur (8).
However, with the growing interest that mpMRI is gaining as a complementary tool for appropriate selection of candidates for active surveillance, there is a strong need of a standardised approach to reporting prostate MRI scans. This has been recently highlighted by the panel of experts who drafted the Prostate Cancer Radiological Estimation of Change in Sequential Evaluation (PRECISE) recommendations, in order to set out the ideal reporting standards for mpMRI in men on active surveillance (63).
Together with standard mpMRI sequences—that allow a qualitative assessment of prostate cancer—a quantitative approach using imaging biomarkers (64,65), such as ADC or texture analysis, holds promise for the detection of change in men on active surveillance for prostate cancer.
However, one of the biggest limitations lies in the conduct of mpMRI exams, as they differ across centres and vendors, (e.g., different b values for DWI, controversial use of spectroscopy, different temporal resolution for DCE, etc.). This heterogeneity in MRI techniques, as well as varying inclusion criteria, makes comparison across different studies challenging.
In conclusion, there is compelling evidence to support the use of mpMRI in men suitable for active surveillance. However, there is still need of robust data from large studies that can investigate its role in the management of men on active surveillance, analysing the huge amount of quantitative data that can be extrapolated from the different mpMRI sequences and investigating the added value of MRI-targeted biopsies to detect clinically significant cancer with the minor number of cores taken.
Acknowledgements
F Giganti is funded by the UCL Graduate Research Scholarship.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015. JAMA Oncol 2017;3:524. [Crossref] [PubMed]
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014;384:2027-35. [Crossref] [PubMed]
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014;370:932-42. [Crossref] [PubMed]
- Carroll PR, Parsons JK, Andriole G, et al. NCCN Guidelines Insights: Prostate Cancer Early Detection, Version 2.2016. J Natl Compr Canc Netw 2016;14:509-19. [Crossref] [PubMed]
- Bruinsma SM, Bangma CH, Carroll PR, et al. Active surveillance for prostate cancer: A narrative review of clinical guidelines. Nat Rev Urol 2016;13:151-67. [Crossref] [PubMed]
- Dall’Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol 2012;62:976-83. [Crossref] [PubMed]
- Selvadurai ED, Singhera M, Thomas K, et al. Medium-term outcomes of active surveillance for localised prostate cancer. Eur Urol 2013;64:981-7. [Crossref] [PubMed]
- Schoots IG, Petrides N, Giganti F, et al. Magnetic resonance imaging in active surveillance of prostate cancer: A systematic review. Eur Urol 2015;67:627-36. [Crossref] [PubMed]
- Itatani R, Namimoto T, Atsuji S, et al. Negative predictive value of multiparametric MRI for prostate cancer detection: outcome of 5-year follow-up in men with negative findings on initial MRI studies. Eur J Radiol 2014;83:1740-5. [Crossref] [PubMed]
- Vargas HA, Akin O, Afaq A, et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol 2012;188:1732-8. [Crossref] [PubMed]
- Fradet V, Kurhanewicz J, Cowan JE, et al. Prostate cancer managed with active surveillance: role of anatomic MR Imaging and MR Spectroscopic Imaging. Radiology 2010;256:176-83. [Crossref] [PubMed]
- Park BH, Jeon HG, Choo SH, et al. Role of multiparametric 3.0-Tesla magnetic resonance imaging in patients with prostate cancer eligible for active surveillance. BJU Int 2014;113:864-70. [Crossref] [PubMed]
- Moore CM, Robertson NL, Arsanious N, et al. Image-guided prostate biopsy using magnetic resonance imaging - derived targets : a systematic review. Eur Urol 2013;63:125-40. [Crossref] [PubMed]
- Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate : recommendations from an international working group. Eur Urol 2013;64:544-52. [Crossref] [PubMed]
- Wegelin O, van Melick HHE, Hooft L, et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies : a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is There a Preferred Technique ? Eur Urol 2017;71:517-31. [Crossref] [PubMed]
- De Visschere PJL, Briganti A, Fütterer JJ, et al. Role of multiparametric magnetic resonance imaging in early detection of prostate cancer. Insights Imaging 2016;7:205-14. [Crossref] [PubMed]
- Giganti F, Moore CM. A critical comparison of techniques for MRI-targeted biopsy of the prostate. Transl Androl Urol 2017;6:432-43. [Crossref] [PubMed]
- Matsugasumi T, Baco E, Palmer S, et al. Prostate cancer volume estimation by combining magnetic resonance imaging and targeted biopsy proven cancer core length: Correlation with cancer volume. J Urol 2015;194:957-65. [Crossref] [PubMed]
- Dianat SS, Carter HB, Pienta KJ, et al. Magnetic resonance-invisible versus magnetic resonance-visible prostate cancer in active surveillance: A preliminary report on disease outcomes. Urology 2015;85:147-53. [Crossref] [PubMed]
- Margel D, Yap SA, Lawrentschuk N, et al. Impact of multiparametric endorectal coil prostate magnetic resonance imaging on disease reclassification among active surveillance candidates: A prospective cohort study. J Urol 2012;187:1247-52. [Crossref] [PubMed]
- Coakley F V., Oto A, Alexander LF, et al. ACR Appropriateness Criteria ® Prostate Cancer—Pretreatment Detection, Surveillance, and Staging. J Am Coll Radiol 2017;14:S245-57. [Crossref] [PubMed]
- Mullins JK, Carter HB. Multiparametric magnetic resonance imaging (MRI) and active surveillance for prostate cancer: Future directions. BJU Int 2014;113:844-5. [Crossref] [PubMed]
- Bruinsma SM, Roobol MJ, Carroll PR, et al. Expert consensus document: Semantics in active surveillance for men with localized prostate cancer-results of a modified Delphi consensus procedure. Nat Rev Urol 2017;14:312-22. [Crossref] [PubMed]
- Kayhan A. Multi-parametric MR imaging of transition zone prostate cancer: Imaging features, detection and staging. World J Radiol 2010;2:180. [Crossref] [PubMed]
- Giganti F, Moore CM, Robertson NL, et al. MRI findings in men on active surveillance for prostate cancer: does dutasteride make MRI visible lesions less conspicuous? Results from a placebo-controlled, randomised clinical trial. Eur Radiol 2017;27:4767-74. [Crossref] [PubMed]
- De Cobelli F, Ravelli S, Esposito A, et al. Apparent diffusion coefficient value and ratio as noninvasive potential biomarkers to predict prostate cancer grading: Comparison with prostate biopsy and radical prostatectomy specimen. AJR Am J Roentgenol 2015;204:550-7. [Crossref] [PubMed]
- Somford DM, Hoeks CM, Hulsbergen-van de Kaa CA, et al. Evaluation of diffusion-weighted MR imaging at inclusion in an active surveillance protocol for low-risk prostate cancer. Invest Radiol 2013;48:152-7. [Crossref] [PubMed]
- Chen Z, Zheng Y, Ji G, et al. Accuracy of dynamic contrast-enhanced magnetic resonance imaging in the diagnosis of prostate cancer: systematic review and meta-analysis. Oncotarget 2017;8:77975-89. [PubMed]
- Kobus T, Vos PC, Hambrock T, et al. Prostate cancer aggressiveness: in vivo assessment of MR Spectroscopy and Diffusion-weighted Imaging at 3 T. Radiology 2012;265:457-67. [Crossref] [PubMed]
- Barth BK, Cornelius A, Nanz D, et al. Comparison of image quality and patient discomfort in prostate MRI: pelvic phased array coil vs. endorectal coil. Abdom Radiol (NY) 2016;41:2218-26. [Crossref] [PubMed]
- Baur ADJ, Daqqaq T, Wagner M, et al. T2- and diffusion-weighted magnetic resonance imaging at 3T for the detection of prostate cancer with and without endorectal coil: An intraindividual comparison of image quality and diagnostic performance. Eur J Radiol 2016;85:1075-84. [Crossref] [PubMed]
- Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746-57. [Crossref] [PubMed]
- Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69:16-40. [Crossref] [PubMed]
- Spektor M, Mathur M, Weinreb JC. Standards for MRI reporting—the evolution to PI-RADS v 2.0. Transl Androl Urol 2017;6:355-67. [Crossref] [PubMed]
- Rosenkrantz AB, Kim S, Lim RP, et al. Prostate cancer localization using multiparametric MR imaging: Comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert scales. Radiology 2013;269:482-92. [Crossref] [PubMed]
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. [Crossref] [PubMed]
- NICE. Prostate cancer: protocol for active surveillance. Implementing the NICE guideline on prostate cancer (CG175). 2014;(January). Available online: https://www.nice.org.uk/guidance/cg175/resources/cg175-prostate-cancer-protocol-for-active-surveillance2
- Tran GN, Leapman MS, Nguyen HG, et al. Magnetic resonance imaging-ultrasound fusion biopsy during prostate cancer active surveillance. Eur Urol 2017;72:275-81. [Crossref] [PubMed]
- Giganti F, Gambarota G, Moore CM, et al. Prostate cancer detection using quantitative T2 and T2 -weighted imaging: The effects of 5-alpha-reductase inhibitors in men on active surveillance. J Magn Reson Imaging 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Moore CM, Robertson NL, Jichi F, et al. The effect of dutasteride on magnetic resonance imaging defined prostate cancer: MAPPED—A Randomized, Placebo Controlled, Double-Blind Clinical Trial. J Urol 2017;197:1006-13. [Crossref] [PubMed]
- Yim JH, Kim CK, Kim JH. Clinically insignificant prostate cancersuitable for active surveillance accordingto prostate cancer research international:active surveillance criteria: Utility ofPI-RADS v2. J Magn Reson Imaging 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Tamada T, Dani H, Taneja SS, et al. The role of whole-lesion apparent diffusion coefficient analysis for predicting outcomes of prostate cancer patients on active surveillance. Abdom Radiol (NY) 2017;42:2340-5. [Crossref] [PubMed]
- Nougaret S, Robertson N, Golia Pernicka J, et al. The performance of PI-RADSv2 and quantitative apparent diffusion coefficient for predicting confirmatory prostate biopsy findings in patients considered for active surveillance of prostate cancer. Abdom Radiol (NY) 2017;42:1968-74. [Crossref] [PubMed]
- Nguyen C, Sharif-Afshar AR, Fan Z, et al. 3D high-resolution diffusion-weighted MRI at 3T: Preliminary application in prostate cancer patients undergoing active surveillance protocol for low-risk prostate cancer. Magn Reson Med 2016;75:616-26. [Crossref] [PubMed]
- Kim TH, Jeong JY, Lee SW, et al. Diffusion-weighted magnetic resonance imaging for prediction of insignificant prostate cancer in potential candidates for active surveillance. Eur Radiol 2015;25:1786-92. [Crossref] [PubMed]
- Jeong CW, Park YH, Hwang SI, et al. The role of 3-tesla diffusion-weighted magnetic resonance imaging in selecting prostate cancer patients for active surveillance. Prostate Int 2014;2:169-75. [Crossref] [PubMed]
- Rosenkrantz AB, Prabhu V, Sigmund EE, et al. Utility of diffusional kurtosis imaging as a marker of adverse pathologic outcomes among prostate cancer active surveillance candidates undergoing radical prostatectomy. AJR Am J Roentgenol 2013;201:840-6. [Crossref] [PubMed]
- Lee DH, Koo KC, Lee SH, et al. Tumor lesion diameter on diffusion weighted magnetic resonance imaging could help predict insignificant prostate cancer in patients eligible for active surveillance: Preliminary analysis. J Urol 2013;190:1213-7. [Crossref] [PubMed]
- Vasarainen H, Lahdensuo K, Savolainen R, et al. Diffusion-weighted magnetic resonance imaging in prostate cancer patients on active surveillance one year after diagnosis and before repeat biopsy. Scand J Urol 2013;47:456-61. [Crossref] [PubMed]
- Giles SL, Morgan VA, Riches SF, et al. Apparent diffusion coefficient as a predictive biomarker of prostate cancer progression: Value of fast and slow diffusion components. AJR Am J Roentgenol 2011;196:586-91. [Crossref] [PubMed]
- Morgan VA, Riches SF, Thomas K, et al. Diffusion-weighted magnetic resonance imaging for monitoring prostate cancer progression in patients managed by active surveillance. Br J Radiol 2011;84:31-7. [Crossref] [PubMed]
- van As NJ, de Souza NM, Riches SF, et al. A study of diffusion-weighted magnetic resonance imaging in men with untreated localised prostate cancer on active surveillance. Eur Urol 2009;56:981-7. [Crossref] [PubMed]
- Bokhorst LP, Valdagni R, Rannikko A, et al. A Decade of active surveillance in the PRIAS Study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur Urol 2016;70:954-60. [Crossref] [PubMed]
- Morgan VA, Parker C, MacDonald A, et al. Monitoring tumor volume in patients with prostate cancer undergoing active surveillance: Is MRI apparent diffusion coefficient indicative of tumor growth? AJR Am J Roentgenol 2017;209:620-8. [Crossref] [PubMed]
- Flavell RR, Westphalen AC, Liang C, et al. Abnormal findings on multiparametric prostate magnetic resonance imaging predict subsequent biopsy upgrade in patients with low risk prostate cancer managed with active surveillance. Abdom Imaging 2014;39:1027-35. [Crossref] [PubMed]
- deSouza NM, Riches SF, VanAs NJ, et al. Diffusion-weighted magnetic resonance imaging: a potential non-invasive marker of tumour aggressiveness in localized prostate cancer. Clin Radiol 2008;63:774-82. [Crossref] [PubMed]
- Sanguedolce F, Petralia G, Sokhi H, et al. Baseline multiparametric mri for selection of prostate cancer patients suitable for active surveillance: which features matter? Clin Genitourin Cancer 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Leapman MS, Westphalen AC, Ameli N, et al. Association between a 17-gene genomic prostate score and multi-parametric prostate MRI in men with low and intermediate risk prostate cancer (PCa). PLoS One 2017;12:e0185535. [Crossref] [PubMed]
- Park JJ, Park BK. Role of PI-RADSv2 with multiparametric MRI in determining who needs active surveillance or definitive treatment according to PRIAS. J Magn Reson Imaging 2017;45:1753-9. [Crossref] [PubMed]
- Hashimoto T, Rahul K, Takeda T, et al. Prostate magnetic resonance imaging findings in patients treated for testosterone deficiency while on active surveillance for low-risk prostate cancer. Urol Oncol 2016;34:530.e9-530.e14. [Crossref] [PubMed]
- Almeida GL, Petralia G, Ferro M, et al. Role of multi-parametric magnetic resonance image and PIRADS score in patients with prostate cancer eligible for active surveillance according PRIAS Criteria. Urol Int 2016;96:459-69. [Crossref] [PubMed]
- Turkbey B, Rastinehad AR, Linehan WM, et al. Prostate cancer : can identify patients who are candidates for active surveillance. Radiology 2013;268:144-52. [Crossref] [PubMed]
- Moore CM, Giganti F, Albertsen P, et al. Reporting magnetic resonance imaging in men on active surveillance for prostate cancer: The PRECISE Recommendations—A Report of a European School of Oncology Task Force. Eur Urol 2017;71:648-55. [Crossref] [PubMed]
- O’Connor JPB, Aboagye EO, Adams JE, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 2017;14:169-86. [Crossref] [PubMed]
- Glaser ZA, Gordetsky JB, Porter KK, et al. Prostate cancer imaging and biomarkers guiding safe selection of active surveillance. Front Oncol 2017;7:256. [Crossref] [PubMed]


