Review of Prader-Willi syndrome: the endocrine approach
Introduction
Prader-Willi syndrome (PWS) is a complex genetic disorder with implications on the endocrine and neurologic systems, metabolism, and behavior. The estimated prevalence of PWS is 1 in 10,000 to 30,000, with equal numbers of male and females affected. PWS results from lack of expression of the paternally-inherited genes on chromosome 15q11.2-q13 due to paternal deletion, maternal uniparental disomy, or imprinting defect of this region (1). Early in life, PWS is characterized by hypotonia and failure to thrive, followed by obesity and hyperphagia (2). Patients with PWS develop hypothalamic dysfunction which may lead to several endocrinopathies, including growth hormone deficiency (GHD), hypogonadism, hypothyroidism, central adrenal insufficiency (CAI), and poor bone mineral density (BMD). In addition to hypothalamic dysfunction and lack of satiety, individuals with PWS have lower resting energy expenditure. Thus, increased risk for obesity which may be complicated by metabolic syndrome and type 2 diabetes mellitus (T2DM) (3). Therefore, individuals with PWS require close management with an endocrinologist throughout their lifespan, from infancy to adulthood. At our institution, we utilize a multi-disciplinary approach to address the complex needs of PWS patients in which individuals see multiple specialists including genetics, neurology, psychology, and dietetics, with endocrinology at the forefront. In this paper, we review the current literature pertaining to the endocrine concerns of the infant, child, adolescent, and adult with PWS and current recommendations for screening and management of these conditions.
Growth hormone (GH)
The GH axis is the most extensively studied within the PWS population and GHD is the most commonly reported endocrinopathy. Although the prevalence of GHD in PWS is reported to range between 40–100% based on various studies (4,5), an altered GH-IGF-1 axis is thought to be a universal feature of this syndrome. The KIGS database (Kabi International Growth Study) noted GHD in 74% and IGF-1 deficiency in nearly 100% of the PWS patients studied (6). Reasons for such discrepancy in prevalence of GHD is likely due to variation in the testing methods used, differences in growth hormone (GH) assays, and age of patients at time of testing.
The exact pathogenesis of GHD in PWS is not entirely clear. PWS children demonstrate decreased spontaneous 24-hour GH secretion, in addition to inadequate response to GH stimulation testing (5). Serum IGF-1 levels are universally noted to be sub-normal as well. It is likely that there is a combination of subnormal GH reserve and IGF-1 generation in individuals with PWS leading to subnormal growth response. Pituitary GH reserve may progressively decline with age in PWS children and GH levels are noted to be relatively lower in obese PWS patients than those who are non-obese (7). Additionally, one study has shown genotypic differences in GH secretary responses on GH stimulation testing, with higher GH response in PWS patients with paternal deletion of 15q11-q13 than in those with maternal UPD15 (8). However, the clinical response to GH treatment based on genotype is not fully defined.
The clinical presentation and features in PWS individuals are consistent with those typically seen in GHD. PWS may present with short stature, growth deceleration, reduced pubertal growth spurt, and short final adult height. Multiple studies have reported improvements not only in linear growth, but various other parameters including: body composition and lean muscle mass, motor development, energy expenditure, BMD and cardiovascular health across all ages of PWS children, adolescents, and adults treated with GH (9-12). Additionally, these positive therapeutic effects have been noted in all patients with PWS irrespective of their baseline GH status. It has been reported that up to 63–74% of patients with PWS have pituitary morphological abnormalities on brain MRI, although there is no direct correlation with pituitary hormone deficiencies (13,14). PWS patients do not need formal testing to demonstrate GHD prior to initiation of GH therapy in the United States (US), yet testing to demonstrate GHD may be still required in some countries (15,16). The US Food and Drug Administration approved GH therapy for PWS in the US in 2000. Current recommendations are for early age of initiation of GH therapy, preferably before onset of obesity, which is often noted to occur by 2 years of age. However, many experts choose to start GH therapy soon after a diagnosis of PWS is made, as early as 3–6 months of age (15,16) given reported improvements in meeting motor and cognitive developmental milestones (9).
The majority of clinical trials examining benefits of GH therapy have utilized a dose of 1 mg/m2/day (15), which is equivalent to about 0.035 mg/kg/day or 0.245 mg/kg/week (17). Clinical guidelines recommend starting GH therapy in infants and children at a lower dose of 0.5 mg/m2/day to avoid potential side effects, then gradually titrating the dose towards 1 mg/m2/day based on IGF-1 (15). Even at these lower doses, PWS patients are noted to be very sensitive to the IGF-1 generation response to GH, and GH treatment often results in IGF-1 levels above the normal reference range (18). Recommendations for frequency of IGF-1 monitoring vary, but in our clinical practice, we obtain IGF-1 levels 1–2 months after starting treatment, then every 6–12 months. In patients that have had an elevated IGF-1 in the past, we recommend checking 1–2 months after a dose increase as well.
Overall, GH therapy is generally safe and well tolerated in children and adolescents with PWS (Figure 1). However, because of reports of unexpected mortality in PWS patients on GH treatment, extreme caution needs exercised for concerns of breathing problems and sleep apnea (19). Polysomnography should be obtained prior to initiating treatment, within 3–6 months after starting GH therapy, and then annually. Additionally, if there is concern for development of snoring or other symptoms suggestive of sleep disordered breathing or sleep apnea, we recommend holding GH therapy until a sleep study can urgently be performed. Lymphoid hyperplasia may theoretically develop due to GH treatment and supra-physiologic IGF-1 levels (20). Expert opinion recommends maintaining IGF-1 in the upper range of normal for appropriate reference ranges, and titrating GH dose based on IGF-1 levels (15).
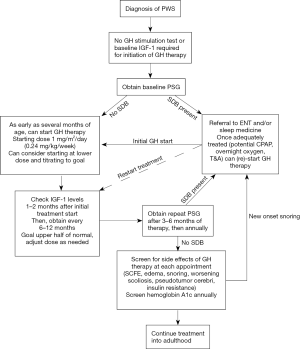
Hemoglobin A1c should be monitored annually during GH treatment due to GH effects on insulin resistance and risks of diabetes and metabolic syndrome in PWS patients. Other potential side effects including joint pain, edema, scoliosis, SCFE, and pseudotumor cerebri should all be closely monitored as in other patients on treatment with GH. PWS patients on GH therapy should preferably be monitored closely by endocrinologists with expertise in PWS. Treatment is generally continued until completion of linear growth. GH treatment then may be transitioned to adult GH treatment based on current guidelines. Usual dose recommendations for GH in adults vary in range of 0.2–1.6 mg/day. IGF-1 levels should be maintained within the upper half of the reference range. Evidence for beneficial effects of adult GH treatment in PWS is growing, and may include improved quality of life, as well as improvements in behavioral and psychologic functioning (15).
Hypogonadism
All patients with PWS have some degree of hypogonadism, although both hypogonadotropic hypogonadism and primary gonadal failure have been reported. In males and females, there is heterogeneity as to the phenotype of hypogonadism (primary or central) in each PWS patient. However, it is usually not correlated to the genetic subtype of PWS (21,22).
Males
Cryptorchidism (either unilateral or bilateral) is almost universal in males with PWS, ranging from 66% to 100% of newborns (23,24), and almost all require orchiopexy. Human chorionic gonadotropin (hCG) treatment has been used to lower the testicular position in most patients, yet almost all patients still required orchiopexy. However, early treatment with hCG may result in better outcomes including development of the scrotal sac and growth of phallus length (25). Most studies report normal penile length at birth and in early childhood (22,26). However, over time, penile length starts to fall below −2SDs compared to healthy males (27). Smaller penile size coupled with a large supra-pubic fat pad seen in many obese PWS patients may lead to difficulty urinating while standing in young males, and treatment with a short course of low-dose testosterone may be considered to aid in potty-training.
PWS males demonstrate a normal mini-puberty of infancy with normally elevated LH, FSH, and testosterone levels within the first few months of life (26). After the mini-puberty, testosterone and the gonadotropins decrease to pre-pubertal levels. At the onset of puberty, testosterone levels increase, but remain low. LH and FSH are more variable, but most studies report that LH levels remain low-normal to normal, while FSH increases and remains normal to high (21-23,27-30). Additionally, most studies report a normal age of onset of puberty in males, although an arrest of pubertal progression typically occurs at Tanner stage 3, coinciding with testicular failure. Testicular size may progress to a volume of 6–7 milliliters and remain small into adulthood (21-23,27-29). PWS males are generally thought to be infertile, and there have been no known reports of PWS males fathering children (31). Inhibin B, a marker of spermatogenesis and Sertoli cell function, is low or undetectable in most adolescents and adults, which is when testicular failure is most evident (21-23,27-30).
No consensus exists as to the most appropriate regimen for pubertal induction or hormone replacement in males with PWS. However, experts agree that the dosing and timing should reflect the normal process of puberty. Typically, treatment with testosterone replacement is recommended for PWS males with delayed or incomplete puberty, usually by age 15–16 years (21,22). At our institution, we observe for natural progression of puberty, but testosterone treatment has been started as early as age 14 years if there is evidence of pubertal stalling. We generally recommend intramuscular testosterone replacement starting at a dose of 50–100 mg given every 28 days with gradual increase towards typical adult male doses. Careful monitoring of growth and skeletal maturation is needed to avoid unfavorable effects of final height. Once males are at adult doses, other forms of testosterone administration can be considered including testosterone patches or gel, although caution must be taken in individuals with skin picking tendencies. Since these patients are already at risk of behavior issues and aggressive outbursts, families should be counseled on the potential increase in these behaviors with testosterone treatment.
Females
Females with PWS are also affected by a combination of hypogonadotropic hypogonadism and primary gonadal failure. PWS females are born with hypoplasia of the external genitalia with labia minora and clitoral hypoplasia in up to 76% (24). The onset of puberty with breast development typically occurs at a normal age, but progression of breast tissue to Tanner 3 and 4 is usually significantly delayed, with very few patients reaching Tanner 5 breast development (32,33). Most females with PWS do not progress to menarche, although 8–25% has spontaneous periods. Average age of reported first period is late but varied, occurring at an average age of 20 years, and almost all have had oligomenorrhea after menarche (21,22,32).
Low to low-normal estrogen and LH levels have been reported in almost all pubertal females with PWS. FSH levels have been found to be more variable in females after pubertal onset (ranging from low to normal to high), which may be indicative of mixed central and primary defects. Inhibin B, a measure of gonadal function, is reported to be low in most adult females with PWS (21,22,30,32-34). However, a subset of PWS females may have preservation of fertility. Studies indicate Inhibin B levels >20 pg/mL correlate with the potential for fertility in PWS females, though these levels are still frankly low (22,34). Although rare, there have been six documented pregnancies in females with PWS. Females with 15q11.2-q13 deletion have a 50% chance of mothering a child with Angelman syndrome (31). Therefore, counseling female patients on reproductive health and contraceptive practices is warranted in all females with PWS.
As in males, there are no formal guidelines for hormone replacement in females with PWS. Our practice has been to monitor females clinically for spontaneous initiation and progression of puberty. If no breast development occurs by age 13 years, pubertal progression stalls, or no menarche by age 16 years then hormone replacement is started. Oral estrogens at graduated doses are typically used for initiation or continuation of stalled puberty, with combined oral contraceptive pills (OCPs) used after the first menstrual bleed has occurred.
Premature adrenarche
There is a high rate of premature adrenarche in both males and females with PWS, with a prevalence of 14–30% (24,35). Androgens levels may be slightly elevated during childhood, but these typically normalize as adults (22,35). GH treatment generally has no effect on adrenarche or DHEA-S levels (35). Adrenarche may be associated with advanced bone age in some cases, although presence of obesity may also be causative of skeletal advancement (36). Premature adrenarche in PWS is typically not rapidly progressive or associated with other signs of central puberty, and is generally felt to be benign. Reassurance should be provided to families that further investigation or treatment is not usually warranted.
Hypothyroidism
Similar to other endocrinopathies in PWS, hypothalamic dysfunction may place patients at increased for central hypothyroidism. There are varying reports as to the prevalence of hypothyroidism in PWS. Some report prevalence to be as common as 20–30% of patients (13), but others have reported it to be present in only 2–4% of the PWS population (37,38), which is not significantly different from healthy controls or the general population.
A 2013 study found no significant difference between TSH and total T4 levels on newborn screening in PWS patients compared to healthy controls. TRH stimulation testing in the same population also did not reveal any cases of tertiary hypothyroidism (38). However, a study examining thyroid function over the first two years of life did report a higher rate of central hypothyroidism in patients with PWS with 13 of 18 patients having low free or total T4 levels, with normal TSH. Therefore, TSH alone or on state newborn screens may not be enough to rule out hypothyroidism in the neonate with PWS (39). Several other studies in older children and adults with PWS have not shown higher rates of central hypothyroidism, and have found similar rates of hypothyroidism compared to healthy controls (37,38).
Thyroid function should be monitored once starting GH therapy. A study by Festen et al. of 79 children with PWS revealed decreases in free T4 after starting GH (although still within the normal range for all but 4 patients). However, TSH remained normal and total T3 levels were normal or high-normal in each patient. This may be due to an increased conversion of T4 to T3, which can be caused by the GH therapy (40).
Although there is some literature showing a higher rate of hypothyroidism at a young age, CNS maturation may help normalize thyroid function as these patients get older (39). Regardless, since there may be an increased prevalence in a patient population already at risk of developmental delays and poor growth, the expert consensus is to screen for hypothyroidism within the first three months of life, and then yearly, especially if on GH therapy (40,41). Treatment with levothyroxine at typical replacement doses based on age and weight should be used if thyroid function is indicative of hypothyroidism.
Central adrenal insufficiency
Individuals with PWS are at risk for CAI due to known hypothalamic dysfunction. However, the true prevalence of CAI in the PWS population is unclear. Several studies using different testing methods including insulin tolerance test (ITT), low dose/high dose ACTH stimulation, glucagon stimulation and overnight metyrapone tests have reported strikingly differing results. The reported prevalence of CAI has ranged from 0% to as high as 60% of the PWS population showing evidence of cortisol deficiency based on these various means of dynamic testing (42-47). This data has been problematic for clinicians, and subsequently, there is no consensus amongst endocrinologists or experts within the field regarding best practice or management.
It has been speculated that CAI may in part be responsible for the high mortality rate of 3% per year reported in PWS patients (48). In a review of 64 cases of death in children with PWS, deaths most frequently occurred due to respiratory insufficiency or sudden death during sleep (49). It is unclear if this high rate of mortality is explained by cardiopulmonary arrest due to undiagnosed and untreated central adrenal insufficiency. Autopsies of PWS individuals with unexplained death have found small adrenal glands by weight, supporting this potential hypothesis (50,51).
In 2008, de Lind van Wijngaarden et al. reported an alarming 60% of children with PWS demonstrated CAI based on ACTH response to overnight metyrapone testing (52). Morning and diurnal cortisol values profiles were found to be normal in these children, despite abnormal ACTH response to metyrapone, suggesting that adrenal insufficiency occurs only during stressful conditions. These authors further suggest that insufficient ACTH response during stress in combination with central sleep apnea during sleep might be responsible for increased risk of sudden death. Subsequent studies have not supported this high rate of CAI in individuals with PWS. Studies using low dose ACTH stimulation tests have shown prevalence of CAI to be an as low as 0–14% (42-44), while testing with ITT and glucagon stimulation test has revealed an even lower prevalence at 0–5% (45-47). Beauloye et al. further studied the relationship between peak cortisol and cortisol increase on ITT and glucagon stimulation test with only one of 20 subjects (5%) showing insufficient response to ITT. Furthermore, these authors did not find significant correlations between cortisol response and central apnea index on polysomnography, which authors suggest do not support a causal link between CAI and central apnea leading to death (47).
Whether or not GH treatment clinically impacts adrenal function in individuals with PWS is currently unknown. GH inhibits 11 β-hydroxysteroid dehydrogenase type 1, decreasing conversion of cortisone to the active hormone cortisol. Treatment with GH, therefore, may theoretically impair optimal adrenal function at baseline and during times of stress. However, Tauber et al. found no differences in causes of death between those PWS children who received GH and those that did not, possibly dismissing this theoretical concern (49). This is an area of study that has not been well-explored and warrants further investigation.
As a result of discrepancies in multiple studies assessing for presence of CAI, there are currently no guidelines or recommendations on the appropriate evaluation and management of CAI in the PWS population. Therefore, clinicians should maintain a high index of suspicion for CAI across the life-span in PWS individuals. Empiric treatment with glucocorticoids during anesthesia or major surgery is recommended by some (53), while others recommended stress dose steroids for all patients during physical stress or illness, including mild upper respiratory infections (52). We would recommend to educate all families on the risk for CAI given hypothalamic dysfunction, and to counsel of signs and symptoms of this condition; although it is notable to mention that individuals with PWS rarely develop fever or signs of illness including vomiting. Our practice has been to assess for CAI by routine testing methods prior to any major surgery or procedure requiring anesthesia and to treat with perioperative steroids empirically if normal adrenal function has not been documented. If CAI is detected, we recommend typical glucocorticoid replacement of 30–50 mg/m2/day during mild or moderate illness divided three times daily, and 75–100 mg/m2/dose given immediately prior to major surgery or anesthesia. Caution should be used to avoid over treatment in a population already at risk for obesity and poor BMD. It is unclear which test is optimal for assessment of CAI in patients with PWS. Additionally, the need for ongoing or repeat testing over the lifetime is uncertain.
Nutritional management and obesity
Traditionally, obesity has been a hallmark feature of PWS. Lean body mass is decreased, even in very young infants and toddlers with normal or non-obese BMI (54). This leads to increased fat mass and lower resting energy expenditure, further promoting weight gain and abnormal body composition (55). Failure to thrive and difficulty feeding due to poor suck is seen during the first year, followed by a period of relative normal appetite and weight gain in the early toddler years. Increased weight gain without an increase in caloric intake occurs by age two to four years, with evidence of increasing appetite and hyperphagic behaviors becoming prominent by school age (56). Hyperphagia and lack of satiety leading to increased risk for obesity predominates during childhood through adulthood, although some but not all adults may develop satiety and resolution of food seeking tendencies. Nutrition counseling therefore is key in preventing excess weight gain, and studies have shown that with early dietary intervention, normal body mass can be achieved during childhood (57).
Study of orexigenic and anorexigenic hormones has been of great interest within the PWS population recently, possibly aiming to explain the stages of appetite dysregulation that are seen throughout infancy, childhood, and into adulthood. The orexigenic hormone ghrelin, which is normally released from the gut during fasting and starvation, has been found to be elevated in PWS children, and even precedes the onset of obesity (58,59). Additionally, in patients with PWS, ghrelin levels remain elevated and do not appropriately suppress after eating when compared to obese controls (60). It is speculated that hyperghrelinemia in early infancy might be a response to failure to thrive, and that chronic or persistent hyperghrelinemia eventually promotes hyperphagia in early childhood.
Anorexigenic hormones including pancreatic polypeptide (PP) are reduced in children with PWS, and peptide YY (PYY) has shown to decline with age (61). However, no differences were found in post-prandial PYY levels in PWS children compared to controls (59), possibly suggesting that regulation of these appetite and satiety peptides might be something that progressively deteriorates over time. Leptin, a hormone secreted by adipocytes to signal satiety, is elevated in children with PWS, suggesting excess fat mass for age and size, decreased lean muscle mass, and a BMI that underestimates the degree of adiposity (61). Recently, studies have investigated the effects of the anorexigenic hormone oxytocin on food-related and social behaviors in patients with PWS with mixed results. While the majority of studies have not shown significant improvement in food-related behaviors (62,63), one study did show improvement in oral feeding skills in very young infants receiving a short course of oxytocin within the first 6 months of life (64), possibly indicating that early administration of oxytocin shows promise in modifying the natural history of feeding and feeding-behaviors in the PWS population. Future studies investigating the relationship of these hormones on appetite and feeding behaviors to potentially guide pharmacologic treatment are needed.
Long-term observational studies reviewing treatment with GH shows improved body mass composition and reduction in BMI, although GH treated PWS individuals are still obese (65). In 2016, Butler et al. published growth curves for GH treated infants and children with PWS. Patients were treated with GH for at least 40% of the lifespan (66). Although their study does not show complete normalization of weight or BMI status in males or females, a downward shift of these profiles when compared to non-GH treated PWS individuals is evident (67).
The development of metabolic syndrome and complications of obesity in PWS children and adults is largely dependent on obesity status. Frequency of insulin resistance, hyperlipidemia, and hypertension in obese PWS children is similar to obese controls, with these conditions being quite rare in non-obese PWS children (68). Subsequent studies have shown obese PWS children to have greater insulin sensitivity and less fatty liver disease compared to obese controls (69,70). These findings have also been reported to persist into adulthood (71). Speculative reasons for this could include abnormal fat composition seen in PWS with increased subcutaneous fat and decreased visceral fat deposition, as well as increased adiponectin, which may serve as a protective factor for development of metabolic syndrome. GH treatment can decrease insulin sensitivity regardless of obesity status, although changes to hemoglobin A1c have not been demonstrated in studies over the short term (72).
Although many individuals with PWS are able to maintain healthier BMI with earlier diagnosis, treatment, and intervention, T2DM is still very common in up to 25% of PWS adults (73), although rarely develops during the childhood years. Recommendations for screening for T2DM and metabolic syndrome are similar to the general guidelines in obese adolescents. Starting around the typical age of puberty, patients with PWS should have hemoglobin A1c, lipid profile, and transaminases obtained for initial surveillance, with yearly screening thereafter in patients that are obese. All PWS patients on GH therapy, even non-obese, should have annual hemoglobin A1c screening. Treatment for T2DM or insulin resistance in PWS should also follow routine guidelines for the general population (41). Intensive dietary counseling and regular exercise are the first-line approach. Metformin can be added as adjunctive therapy, and has been shown to have positive effects on satiety in some, but not all children and adolescents (74). The addition of insulin is also routinely needed based on hemoglobin A1c (75). More recently, treatment with GLP-1 agonists (exenatide, liraglutide) has been shown to have beneficial effects on weight, satiety, and glycemic control in PWS adults with T2DM (76-80). These medications have been well-tolerated (less bloating, nausea, and vomiting) in PWS patients, possibly due to higher threshold for pain and nausea in PWS (76,77); however, caution must be used due to potential side effect of delayed gastric emptying and pre-existing risk for gastric rupture in the population (76,81).
Bone health/vitamin D deficiency
Historically, it has been reported that osteoporosis and increased fractures are seen at increased frequencies in PWS. Fracture rates have been reported as high as 29% in adults with PWS (73). Many of the earlier studies examined BMD in adults with PWS, reporting significantly lower total BMD and high rates of osteoporosis in 60–90% (82-84). However, more recent studies in PWS children show no differences in BMD when compared to controls (85-87). BMD seems to decline during adolescent years, by age 11 in females and age 14 in males (85), and will eventually decline to osteoporosis by adulthood (82-84). Younger age of initiation of GH treatment has been associated with improved adolescent BMD (85), however, starting GH treatment in adulthood has not shown similar improvements in BMD (88). Most likely, the decrease in BMD during adolescence and osteoporosis in adulthood in PWS patients is due to hypogonadism and lower sex hormones during this time (85,86). 25(OH)-vitamin D levels in PWS adults are similar to the general population (89), although studies in PWS children have shown that 12% have 25(OH)-vitamin D levels less than 20 ng/mL, and 49% have levels less than 30 ng/mL (87). Guidelines for optimal 25(OH)-vitamin D in the general population vary, although most agree that levels less than 20 ng/mL are deficient/insufficient (90-92). Edouard et al. noted that calcium, phosphorus, alkaline phosphatase levels, and radiological findings were similar in children with PWS, regardless of whether 25(OH)-vitamin D levels were sufficient (defined as greater than 20 ng/mL) or insufficient (87). There are no specific guidelines for optimal 25(OH)-vitamin D levels in the PWS population, and it remains unclear how hypovitaminosis D may play a role in BMD decline into adulthood.
There are no clear recommendations regarding monitoring bone health in PWS patients, although assessment of BMD with bone densitometry scan around the age of puberty and every 1–2 years thereafter has been our routine practice. Ensuring normal vitamin D status and normal calcium intake or dietary supplementation for age in light of calorie restriction is needed. We recommend obtaining annual 25(OH)-vitamin D levels. Avoiding delayed treatment of hypogonadism may also help lessen the risk of low BMD and osteoporosis. Further research in this area is needed to improve the guidelines for screening and treatment of low BMD in this population.
Conclusions and future directives
PWS is a complex disorder involving the hypothalamic-pituitary axis, resulting in multiple endocrinopathies, among other issues. Monitoring and management of these conditions in the PWS child, adolescent, and adult is best navigated by endocrinologists experienced with this complex disorder to provide the best care to this population. There is increasing appreciation for the management of PWS in a multi-disciplinary setting. However, it is important for endocrinologists and general pediatricians to be knowledgeable of the screening and monitoring recommendations in PWS (Table 1). In recent years, drastic increases in scientific knowledge of this disease have improved clinical management of this condition, leading to better outcomes for PWS individuals. Future research should be directed at continuing to define clinical guidelines for best practice.

Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cassidy SB, Schwartz S, Miller JL, et al. Prader-Willi syndrome. Genet Med 2012;14:10-26. [Crossref] [PubMed]
- Miller JL. Approach to the child with prader-willi syndrome. J Clin Endocrinol Metab 2012;97:3837-44. [Crossref] [PubMed]
- Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest 2015;38:1249-63. [Crossref] [PubMed]
- Burman P, Ritzén EM, Lindgren AC. Endocrine dysfunction in Prader-Willi syndrome: a review with special reference to GH. Endocr Rev 2001;22:787-99. [Crossref] [PubMed]
- Diene G, Mimoun E, Feigerlova E, et al. Endocrine disorders in children with Prader-Willi syndrome--data from 142 children of the French database. Horm Res Paediatr 2010;74:121-8. [Crossref] [PubMed]
- Tauber M, Cutfield W. KIGS highlights: growth hormone treatment in Prader-Willi Syndrome. Horm Res 2007;68 Suppl 5:48-50. [PubMed]
- Cohen M, Harrington J, Narang I, et al. Growth hormone secretion decreases with age in paediatric Prader-Willi syndrome. Clin Endocrinol (Oxf) 2015;83:212-5. [Crossref] [PubMed]
- Di Giorgio G, Grugni G, Fintini D, et al. Growth hormone response to standard provocative stimuli and combined tests in very young children with Prader-Willi syndrome. Horm Res Paediatr 2014;81:189-95. [Crossref] [PubMed]
- Festen DA, de Lind van Wijngaarden R, van Eekelen M, et al. Randomized controlled GH trial: effects on anthropometry, body composition and body proportions in a large group of children with Prader-Willi syndrome. Clin Endocrinol (Oxf) 2008;69:443-51. [Crossref] [PubMed]
- Myers SE, Whitman BY, Carrel AL, et al. Two years of growth hormone therapy in young children with Prader-Willi syndrome: physical and neurodevelopmental benefits. Am J Med Genet A 2007;143A:443-8. [Crossref] [PubMed]
- Carrel AL, Myers SE, Whitman BY, et al. Benefits of long-term GH therapy in Prader-Willi syndrome: a 4-year study. J Clin Endocrinol Metab 2002;87:1581-5. [Crossref] [PubMed]
- Haqq AM, Stadler DD, Jackson RH, et al. Effects of growth hormone on pulmonary function, sleep quality, behavior, cognition, growth velocity, body composition, and resting energy expenditure in Prader-Willi syndrome. J Clin Endocrinol Metab 2003;88:2206-12. [Crossref] [PubMed]
- Tauber M, Barbeau C, Jouret B, et al. Auxological and endocrine evolution of 28 children with Prader-Willi syndrome: effect of GH therapy in 14 children. Horm Res 2000;53:279-87. [PubMed]
- Miller JL, Goldstone AP, Couch JA, et al. Pituitary abnormalities in Prader-Willi syndrome and early onset morbid obesity. Am J Med Genet A 2008;146A:570-7. [Crossref] [PubMed]
- Deal CL, Tony M, Höybye C, et al. GrowthHormone Research Society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab 2013;98:E1072-87. [Crossref] [PubMed]
- Grugni G, Sartorio A, Crinò A. Growth hormone therapy for Prader-willi syndrome: challenges and solutions. Ther Clin Risk Manag 2016;12:873-81. [Crossref] [PubMed]
- Bakker NE, Kuppens RJ, Siemensma EP, et al. Eight years of growth hormone treatment in children with Prader-Willi syndrome: maintaining the positive effects. J Clin Endocrinol Metab 2013;98:4013-22. [Crossref] [PubMed]
- Feigerlová E, Diene G, Oliver I, et al. Elevated insulin-like growth factor-I values in children with Prader-Willi syndrome compared with growth hormone (GH) deficiency children over two years of GH treatment. J Clin Endocrinol Metab 2010;95:4600-8. [Crossref] [PubMed]
- Van Vliet G, Deal CL, Crock PA, et al. Sudden death in growth hormone-treated children with Prader-Willi syndrome. J Pediatr 2004;144:129-31. [Crossref] [PubMed]
- Miller J, Silverstein J, Shuster J, et al. Short-term effects of growth hormone on sleep abnormalities in Prader-Willi syndrome. J Clin Endocrinol Metab 2006;91:413-7. [Crossref] [PubMed]
- Gross-Tsur V, Hirsch HJ, Benarroch F, et al. The FSH-inhibin axis in prader-willi syndrome: heterogeneity of gonadal dysfunction. Reprod Biol Endocrinol 2012;10:39. [Crossref] [PubMed]
- Hirsch HJ, Eldar-Geva T, Bennaroch F, et al. Sexual dichotomy of gonadal function in Prader-Willi syndrome from early infancy through the fourth decade. Hum Reprod 2015;30:2587-96. [Crossref] [PubMed]
- Eiholzer U, l'Allemand D, Rousson V, et al. Hypothalamic and gonadal components of hypogonadism in boys with Prader-Labhart- Willi syndrome. J Clin Endocrinol Metab 2006;91:892-8. [Crossref] [PubMed]
- Crinò A, Schiaffini R, Ciampalini P, et al. Hypogonadism and pubertal development in Prader-Willi syndrome. Eur J Pediatr 2003;162:327-33. [PubMed]
- Bakker NE, Wolffenbuttel KP, Looijenga LH, et al. Testes in infants with Prader-Willi syndrome: human chorionic gonadotropin treatment, surgery and histology. J Urol 2015;193:291-8. [Crossref] [PubMed]
- Fillion M, Deal CL, Van Vliet G. Normal minipuberty of infancy in boys with Prader-Willi syndrome. J Pediatr 2006;149:874-6. [Crossref] [PubMed]
- Hirsch HJ, Eldar-Geva T, Benarroch F, et al. Primary testicular dysfunction is a major contributor to abnormal pubertal development in males with Prader-Willi syndrome. J Clin Endocrinol Metab 2009;94:2262-8. [Crossref] [PubMed]
- Siemensma EP, de Lind van Wijngaarden RF, Otten BJ, et al. Testicular failure in boys with Prader-Willi syndrome: longitudinal studies of reproductive hormones. J Clin Endocrinol Metab 2012;97:E452-9. [Crossref] [PubMed]
- Radicioni AF, Di Giorgio G, Grugni G, et al. Multiple forms of hypogonadism of central, peripheral or combined origin in males with Prader-Willi syndrome. Clin Endocrinol (Oxf) 2012;76:72-7. [Crossref] [PubMed]
- Brandau DT, Theodoro M, Garg U, et al. Follicle stimulating and leutinizing hormones, estradiol and testosterone in Prader-Willi syndrome. Am J Med Genet A 2008;146A:665-9. [Crossref] [PubMed]
- Schulze A, Mogensen H, Hamborg-Petersen B, et al. Fertility in Prader-Willi syndrome: a case report with Angelman syndrome in the offspring. Acta Paediatr 2001;90:455-9. [Crossref] [PubMed]
- Siemensma EP, van Alfen-van der Velden AA, Otten BJ, et al. Ovarian function and reproductive hormone levels in girls with Prader-Willi syndrome: a longitudinal study. J Clin Endocrinol Metab 2012;97:E1766-73. [Crossref] [PubMed]
- Eldar-Geva T, Hirsch HJ, Benarroch F, et al. Hypogonadism in females with Prader-Willi syndrome from infancy to adulthood: variable combinations of a primary gonadal defect and hypothalamic dysfunction. Eur J Endocrinol 2010;162:377-84. [Crossref] [PubMed]
- Eldar-Geva T, Hirsch HJ, Pollak Y, et al. Management of hypogonadism in adolescent girls and adult women with Prader-Willi syndrome. Am J Med Genet A 2013;161A:3030-4. [Crossref] [PubMed]
- Siemensma EP, de Lind van Wijngaarden RF, Otten BJ, et al. Pubarche and serum dehydroepiandrosterone sulphate levels in children with Prader-Willi syndrome. Clin Endocrinol (Oxf) 2011;75:83-9. [Crossref] [PubMed]
- Schmidt H, Schwarz HP. Premature adrenarche, increased growth velocity and accelerated bone age in male patients with Prader-Labhart-Willi syndrome. Eur J Pediatr 2001;160:69-70. [Crossref] [PubMed]
- Butler MG, Theodoro M, Skouse JD. Thyroid function studies in Prader-Willi syndrome. Am J Med Genet A 2007;143A:488-92. [Crossref] [PubMed]
- Sharkia M, Michaud S, Berthier MT, et al. Thyroid function from birth to adolescence in Prader-Willi syndrome. J Pediatr 2013;163:800-5. [Crossref] [PubMed]
- Vaiani E, Herzovich V, Chaler E, et al. Thyroid axis dysfunction in patients with Prader-Willi syndrome during the first 2 years of life. Clin Endocrinol (Oxf) 2010;73:546-50. [PubMed]
- Festen DA, Visser TJ, Otten BJ, et al. Thyroid hormone levels in children with Prader-Willi syndrome before and during growth hormone treatment. Clin Endocrinol (Oxf) 2007;67:449-56. [Crossref] [PubMed]
- Goldstone AP, Holland AJ, Hauffa BP, et al. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab 2008;93:4183-97. [Crossref] [PubMed]
- Grugni G, Beccaria L, Corrias A, et al. Central adrenal insufficiency in young adults with Prader-Willi syndrome. Clin Endocrinol (Oxf) 2013;79:371-8. [Crossref] [PubMed]
- Corrias A, Grugni G, Crinò A, et al. Assessment of central adrenal insufficiency in children and adolescents with Prader-Willi syndrome. Clin Endocrinol (Oxf) 2012;76:843-50. [Crossref] [PubMed]
- Nyunt O, Cotterill AM, Archbold SM, et al. Normal cortisol response on low-dose synacthen (1 microg) test in children with Prader Willi syndrome. J Clin Endocrinol Metab 2010;95:E464-7. [Crossref] [PubMed]
- Farholt S, Sode-Carlsen R, Christiansen JS, et al. Normal cortisol response to high-dose synacthen and insulin tolerance test in children and adults with Prader-Willi syndrome. J Clin Endocrinol Metab 2011;96:E173-80. [Crossref] [PubMed]
- Connell NA, Paterson WF, Wallace AM, et al. Adrenal function and mortality in children and adolescents with Prader-Willi syndrome attending a single centre from 1991-2009. Clin Endocrinol (Oxf) 2010;73:686-8. [Crossref] [PubMed]
- Beauloye V, Dhondt K, Buysse W, et al. Evaluation of the hypothalamic-pituitary-adrenal axis and its relationship with central respiratory dysfunction in children with Prader-Willi syndrome. Orphanet J Rare Dis 2015;10:106. [Crossref] [PubMed]
- Whittington JE, Holland AJ, Webb T, et al. Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J Med Genet 2001;38:792-8. [Crossref] [PubMed]
- Tauber M, Diene G, Molinas C, et al. Review of 64 cases of death in children with Prader-Willi syndrome (PWS). Am J Med Genet A 2008;146A:881-7. [Crossref] [PubMed]
- Stevenson DA, Anaya TM, Clayton-Smith J, et al. Unexpected death and critical illness in Prader-Willi syndrome: report of ten individuals. Am J Med Genet A 2004;124A:158-64. [Crossref] [PubMed]
- Schrander-Stumpel CT, Curfs LM, Sastrowijoto P, et al. Prader-Willi syndrome: causes of death in an international series of 27 cases. Am J Med Genet A 2004;124A:333-8. [Crossref] [PubMed]
- de Lind van Wijngaarden RF, Otten BJ, Festen DA, et al. High prevalence of central adrenal insufficiency in patients with Prader-Willi syndrome. J Clin Endocrinol Metab 2008;93:1649-54. [Crossref] [PubMed]
- Barbara DW, Hannon JD, Hartman WR. Intraoperative adrenal insufficiency in a patient with prader-willi syndrome. J Clin Med Res 2012;4:346-8. [PubMed]
- Eiholzer U, Blum WF, Molinari L. Body fat determined by skinfold measurements is elevated despite underweight in infants with Prader-Labhart-Willi syndrome. J Pediatr 1999;134:222-5. [Crossref] [PubMed]
- Butler MG, Theodoro MF, Bittel DC, et al. Energy expenditure and physical activity in Prader-Willi syndrome: comparison with obese subjects. Am J Med Genet A 2007;143A:449-59. [Crossref] [PubMed]
- Miller JL, Lynn CH, Driscoll DC, et al. Nutritional phases in Prader-Willi syndrome. Am J Med Genet A 2011;155A:1040-9. [Crossref] [PubMed]
- Schmidt H, Pozza SB, Bonfig W, et al. Successful early dietary intervention avoids obesity in patients with Prader-Willi syndrome: a ten-year follow-up. J Pediatr Endocrinol Metab 2008;21:651-5. [Crossref] [PubMed]
- Feigerlová E, Diene G, Conte-Auriol F, et al. Hyperghrelinemia precedes obesity in Prader-Willi syndrome. J Clin Endocrinol Metab 2008;93:2800-5. [Crossref] [PubMed]
- Bizzarri C, Rigamonti AE, Luce A, et al. Children with Prader-Willi syndrome exhibit more evident meal-induced responses in plasma ghrelin and peptide YY levels than obese and lean children. Eur J Endocrinol 2010;162:499-505. [Crossref] [PubMed]
- Gumus Balikcioglu P, Balikcioglu M, Muehlbauer MJ, et al. Macronutrient Regulation of Ghrelin and Peptide YY in Pediatric Obesity and Prader-Willi Syndrome. J Clin Endocrinol Metab 2015;100:3822-31. [Crossref] [PubMed]
- Goldstone AP, Holland AJ, Butler JV, et al. Appetite hormones and the transition to hyperphagia in children with Prader-Willi syndrome. Int J Obes (Lond) 2012;36:1564-70. [Crossref] [PubMed]
- Kuppens RJ, Donze SH, Hokken-Koelega AC. Promising effects of oxytocin on social and food-related behaviour in young children with Prader-Willi syndrome: a randomized, double-blind, controlled crossover trial. Clin Endocrinol (Oxf) 2016;85:979-87. [Crossref] [PubMed]
- Miller JL, Tamura R, Butler MG, et al. Oxytocin treatment in children with Prader-Willi syndrome: A double-blind, placebo-controlled, crossover study. Am J Med Genet A 2017;173:1243-50. [Crossref] [PubMed]
- Tauber M, Boulanouar K, Diene G, et al. The Use of Oxytocin to Improve Feeding and Social Skills in Infants With Prader-Willi Syndrome. Pediatrics 2017;139. [Crossref] [PubMed]
- Sipilä I, Sintonen H, Hietanen H, et al. Long-term effects of growth hormone therapy on patients with Prader-Willi syndrome. Acta Paediatr 2010;99:1712-8. [Crossref] [PubMed]
- Butler MG, Lee J, Cox DM, et al. Growth Charts for Prader-Willi Syndrome During Growth Hormone Treatment. Clin Pediatr (Phila) 2016;55:957-74. [Crossref] [PubMed]
- Butler MG, Lee J, Manzardo AM, et al. Growth charts for non-growth hormone treated Prader-Willi syndrome. Pediatrics 2015;135:e126-35. [Crossref] [PubMed]
- Brambilla P, Crinò A, Bedogni G, et al. Metabolic syndrome in children with Prader-Willi syndrome: the effect of obesity. Nutr Metab Cardiovasc Dis 2011;21:269-76. [PubMed]
- Haqq AM, Muehlbauer MJ, Newgard CB, et al. The metabolic phenotype of Prader-Willi syndrome (PWS) in childhood: heightened insulin sensitivity relative to body mass index. J Clin Endocrinol Metab 2011;96:E225-32. [Crossref] [PubMed]
- Fintini D, Inzaghi E, Colajacomo M, et al. Non-Alcoholic Fatty Liver Disease (NAFLD) in children and adolescents with Prader-Willi Syndrome (PWS). Pediatr Obes 2016;11:235-8. [Crossref] [PubMed]
- Bedogni G, Grugni G, Nobili V, et al. Is non-alcoholic fatty liver disease less frequent among women with Prader-Willi syndrome? Obes Facts 2014;7:71-6. [Crossref] [PubMed]
- Jørgensen AP, Ueland T, Sode-Carlsen R, et al. Glucose homeostasis in adults with Prader-Willi syndrome during treatment with growth hormone: results from a 12-month prospective study. Growth Horm IGF Res 2014;24:16-21. [Crossref] [PubMed]
- Butler JV, Whittington JE, Holland AJ, et al. Prevalence of, and risk factors for, physical ill-health in people with Prader-Willi syndrome: a population-based study. Dev Med Child Neurol 2002;44:248-55. [Crossref] [PubMed]
- Miller JL, Linville TD, Dykens EM. Effects of metformin in children and adolescents with Prader-Willi syndrome and early-onset morbid obesity: a pilot study. J Pediatr Endocrinol Metab 2014;27:23-9. [Crossref] [PubMed]
- Butler MG, Lee PD, Whitman BY. editors. Management of Prader-Willi Syndrome. 3 ed. New York, NY: Springer, 2006.
- Sze L, Purtell L, Jenkins A, et al. Effects of a single dose of exenatide on appetite, gut hormones, and glucose homeostasis in adults with Prader-Willi syndrome. J Clin Endocrinol Metab 2011;96:E1314-9. [Crossref] [PubMed]
- Seetho IW, Jones G, Thomson GA, et al. Treating diabetes mellitus in Prader-Willi syndrome with Exenatide. Diabetes Res Clin Pract 2011;92:e1-2. [Crossref] [PubMed]
- Cyganek K, Koblik T, Kozek E, et al. Liraglutide therapy in Prader-Willi syndrome. Diabet Med 2011;28:755-6. [Crossref] [PubMed]
- Senda M, Ogawa S, Nako K, et al. The glucagon-like peptide-1 analog liraglutide suppresses ghrelin and controls diabetes in a patient with Prader-Willi syndrome. Endocr J 2012;59:889-94. [Crossref] [PubMed]
- Fintini D, Grugni G, Brufani C, et al. Use of GLP-1 receptor agonists in Prader-Willi Syndrome: report of six cases. Diabetes Care 2014;37:e76-7. [Crossref] [PubMed]
- Arenz T, Schwarzer A, Pfluger T, et al. Delayed gastric emptying in patients with Prader Willi Syndrome. J Pediatr Endocrinol Metab 2010;23:867-71. [Crossref] [PubMed]
- Vestergaard P, Kristensen K, Bruun JM, et al. Reduced bone mineral density and increased bone turnover in Prader-Willi syndrome compared with controls matched for sex and body mass index--a cross-sectional study. J Pediatr 2004;144:614-9. [Crossref] [PubMed]
- Butler MG, Haber L, Mernaugh R, et al. Decreased bone mineral density in Prader-Willi syndrome: comparison with obese subjects. Am J Med Genet 2001;103:216-22. [Crossref] [PubMed]
- van Mil EG, Westerterp KR, Gerver WJ, et al. Body composition in Prader-Willi syndrome compared with nonsyndromal obesity: Relationship to physical activity and growth hormone function. J Pediatr 2001;139:708-14. [Crossref] [PubMed]
- Bakker NE, Kuppens RJ, Siemensma EP, et al. Bone mineral density in children and adolescents with Prader-Willi syndrome: a longitudinal study during puberty and 9 years of growth hormone treatment. J Clin Endocrinol Metab 2015;100:1609-18. [Crossref] [PubMed]
- de Lind van Wijngaarden RF, Festen DA, Otten BJ, et al. Bone mineral density and effects of growth hormone treatment in prepubertal children with Prader-Willi syndrome: a randomized controlled trial. J Clin Endocrinol Metab 2009;94:3763-71. [Crossref] [PubMed]
- Edouard T, Deal C, Van Vliet G, et al. Muscle-bone characteristics in children with Prader-Willi syndrome. J Clin Endocrinol Metab 2012;97:E275-81. [Crossref] [PubMed]
- Jørgensen AP, Ueland T, Sode-Carlsen R, et al. Two years of growth hormone treatment in adults with Prader-Willi syndrome do not improve the low BMD. J Clin Endocrinol Metab 2013;98:E753-60. [Crossref] [PubMed]
- Purtell L, Viardot A, Campbell LV. Vitamin D levels in primary growth hormone deficiency disorder Prader-Willi syndrome. Endocrine 2016;53:619-20. [Crossref] [PubMed]
- Ross AC, Manson JE, Abrams SA, et al. The 2011 Dietary Reference Intakes for Calcium and Vitamin D: what dietetics practitioners need to know. J Am Diet Assoc 2011;111:524-7. [Crossref] [PubMed]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911-30. [Crossref] [PubMed]
- Misra M, Pacaud D, Petryk A, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 2008;122:398-417. [Crossref] [PubMed]


