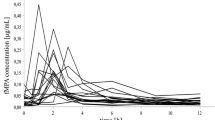
Summary
Cyclosporin is a powerful immunosuppressive drug used in transplantation medicine and to treat autoimmune diseases. It is a lipophilic molecule, with its bioavailability dependent on food, bile and other interacting factors. Cyclosporin is extensively metabolised in the liver by the cytochrome P450 3A system, which is subject to considerable interindividual variation.
Distribution of cyclosporin depends not only on physicochemical characteristics, but also on biological carriers such as lipoproteins and erythrocytes in blood. Cyclophilin, a binding protein for cyclosporin, influences distribution of cyclosporin in the body. Despite its lipophilicity, cyclosporin does not appear in the brain. The distribution of metabolites in the body can differ from that of cyclosporin itself Elimination of the drug is mainly via the bile as metabolites, other routes not being very important.
Pharmacokinetic parameters of cyclosporin are highly variable and depend on factors such as age, the physical condition of the patient, type of organ transplant or comedication. Renal side effects of cyclosporin are dose-related, but the influence of the dosage regimen has not been thoroughly investigated.
An important factor in the reported variability is the different analytical methods used. Following the recommendations of recent consensus documents to monitor blood concentrations, this source of variability may diminish in the future.
Several metabolites are reported as having less immunosuppressive activity than the parent drug. Metabolites with renal side effects have been reported. These and other effects of metabolites have not been clearly defined in the literature, presumably because of the highly variable activity of cyclosporin-metabolising liver enzymes and the paucity of data available on metabolite pharmacokinetics.
The therapeutic range and dosage of cyclosporin are therefore highly dependent on many individual parameters in patients. Dosages of less than 5 mg/kg/day, however, rarely cause renal side effects. Further studies to correlate the clinical pharmacokinetics of metabolites with their activity and adverse effects are needed.
Similar content being viewed by others
References
Adams DH, Ponsford S, Gunson B, Boon A, Honigsberger L, et al. Neurological complications following liver transplantation. Lancet 1: 949–951, 1987
Albrechtsen D, Helgerud P, Jakobsen A, Rugstad HE. Blood and intestinal lymph cyclosporine levels in the rat after oral and intravenous medication. Transplantation Proceedings 18: 42–43, 1986
Atkinson K, Biggs JC, Britton K, Short R, Mrongovius R, et al. Oral administration of cyclosporin A for recipients of allogenic marrow transplants: implications of clinical gut dysfunction. British Journal of Haematology 56: 223–231, 1984
Awni WM, Heim-Duthoy K, Anderson R, Kasiske BL. Time-dependent changes in the pharmacokinetics of cyclosporine and its metabolites in renal transplant patients. Journal of Pharmaceutical Science 76: S63, 1987
Awni WM, Heim-Duthoy KL, Rao KV, Kasiske BL. Pharmacokinetics of cyclosporine and its M1, M17 and M21 metabolites in renal transplant patients over time. Clinical Pharmacology and Therapeutics 43: 198, 1988
Awni MW, Sawchuk RJ. The pharmacokinetics of cyclosporine: I. Single dose and constant rate infusion studies in the rabbit. Drug Metabolism and Disposition 13: 127–132, 1985
Bach JF. Cyclosporine in autoimmune diseases. Transplantation Proceedings 21 (Suppl. 1): 97–113, 1989
Begley DJ, Squires LK, Zlokovic BV, Mitrovic DM, Hughes CCW, et al. Permeability of the blood-brain barrier to the immunosuppressive cyclic peptide cyclosporin A. Journal of Neurochemistry 55: 1222–1230, 1990
Bernareggi B, Rowland M. Physiologic modeling of cyclosporin kinetics in rat and man. Journal of Pharmacokinetics and Biopharmaceutics 19: 21–50, 1991
Beveridge T, Gratwohl A, Michot F, Niederberger W, Nüesch E, et al. Cyclosporin A: pharmacokinetics after a single dose in man and serum levels after multiple dosing in recipients of allogeneic bone-marrow grafts. Current Therapeutic Research 30: 5–18, 1981
Bolas-Fernandez F, Grencis RK, Wakelin D. Cyclosporin A and Trichinella spiralis: anthelmintic effects in immunosuppressed mice. Parasitic Immunology 10: 111–116, 1988
Borel JF, Feurer C, Gubler HU, Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents and Actions 6: 468–475, 1976
Bowers LD, Canafax DM, Singh J, Seifedlin R, Simmons RL, Najarian JS. Studies of cyclosporine blood levels: analysis, clinical utility, pharmacokinetics, metabolites and chronopharmacology. Transplantation Proceedings 18 (Suppl. 5): 137–143, 1986
Burckart GJ, Venkataramanan R, Ptachcinski RJ, Starzl TE, Gartner JC, et al. Cyclosporine absorption following orthotopic liver transplantation. Journal of Clinical Pharmacology 26: 647–651, 1986
Burke MD, Whiting PH. The role of drug metabolism in cyclosporin A nephrotoxicity. Clinical Nephrology 25 (Suppl. 1): S111–S116, 1986
Busuttil RW, Goldstein LI, Danovitch GM, Ament ME, Memsic LDF. UCLA conference: Liver transplantation today. Annals of Internal Medicine 104: 377–389, 1986
Calne RY, Rolles K, White DJG, Thiru S, Evans DB, et al. Cyclosporin A as initially the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases and 2 livers. Lancet 2: 1033–1036, 1979
Calne RY, White DJG, Thiru S, Evans DB, McMaster P, et al. Cyclosporine A in patients receiving renal allografts from cadaver donors. Lancet 2: 1323–1327, 1978
Canafax DM, Cipolle RJ, Hrushesky WJM, Rabatin JT, Min DI, et al. The chronopharmacokinetics of cyclosporine and its metabolites in recipients of pancreas allografts. Transplantation Proceedings 20 (Suppl. 2): 471–477, 1988
Cefalu WT, Pardridge WM. Restrictive transport of a lipid-soluble peptide (cyclosporin) through the blood-brain barrier. Journal of Neurochemistry 45: 1954–1956, 1985
Christians U, Kohlhaw K, Budniak J, Bleck JS, Schottmann R, et al. Ciclosporin metabolite pattern in blood and urine of liver graft recipients I. Association of Ciclosporin metabolites with nephrotoxicity. European Journal of Clinical Pharmacology 41: 285–290, 1991a
Christians U, Schlitt HJ, Bleck JS, Schiebel HM, Kownatzki R, et al. Measurement of cyclosporine and 18 metabolites in blood, bile and urine by high-performance liquid chromatography. Transplantation Proceedings 20 (Suppl. 2): 609–613, 1988
Christians U, Strohmeyer S, Kownatzki R, Schiebel HM, Bleck J, et al. Investigations on the metabolic pathways of cyclosporine: I. Excretion of cyclosporine and its metabolites in human bile: isolation of 12 new cyclosporine metabolites. Xenobiotica 21: 1185–1198, 1991b
Cipolle RJ, Canafax DM, Rabatin J, Bowers LD, Sutherland DER, et al. Time-dependent disposition of cyclosporine after pancreas transplantation, and application of chronopharmacokinetics to improve immunosuppression. Pharmacotherapy 8: 47–51, 1988
Clardy CW, Schroeder TJ, Myre SA, Wadhwa NK, Pesce AJ, et al. Clinical variability of cyclosporine pharmacokinetics in adult and paediatric patients after renal, cardiac, hepatic and bone marrow transplants. Clinical Chemistry 34: 2012–2015, 1988
Combalbert J, Fabre I, Fabre G, Dalet I, Derancourt J, et al. Metabolism of cyclosporin A. IV. Purification and identification of the rifampicin inducible human liver cytochrome P450 (cyclosporin A oxidase) as a product of P450IIIA gene subfamily. Drug Metabolism and Disposition 17: 197–207, 1989
Consensus Document: Hawk’s Cay meeting on therapeutic drug monitoring of cyclosporine. Transplantation Proceedings 22: 1357–1361, 1990
Copeland KR, Yatscoff RW. Use of a monoclonal antibody for the therapeutic monitoring of cyclosporine in plasma and whole blood. Therapeutic Drug Monitoring 10: 453–458, 1988
De Groen PC. Cyclosporine, low-density lipoprotein, and cholesterol. Mayo Clinic Proceedings 63: 1012–1021, 1988
De Groen PC, Aksamit AJ, Rakela J, Forbes GS, Krom RAF. Central nervous system toxicity after liver transplantation: the role of cyclosporine and cholesterol. New England Journal of Medicine 317: 861–866, 1987
Dieperink H, Leyssac PP, Starklint H, Kemp E. Cyclosporine A administration: once a day or in fractional doses?, Transplantation Proceedings 20 (Suppl. 2): 703–706, 1988
Drewe J, Beglinger C, Kissel T. The absorption site of cyclosporin in the human gastrointestinal tract, British Journal of Clinical Pharmacology 33: 39–43, 1992
Edwards DJ, Ducharme MP, Provenzano R, Smith-Dehoorne M. Effect of grapefruit juice on blood concentrations of cyclosporine. Clinical Pharmacology and Therapeutics 53: 237, 1993
Fahr A, Hiestand P, Ryffel B. Studies on the biological activities of Sandimmun metabolites in humans and in animal models: review and original experiments. Transplantation Proceedings 22: 1116–1124, 1990
Faulds D, Goa KL, Benfield P. Cyclosporin: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs, in press, 1993
Ferguson RM, Rynasiewicz JJ, Sutherland DER, Simmons RL, Najarian JS. Cyclosporin A in renal transplantation: a prospective randomized trial. Surgery 92: 175–182, 1982
First MR, Schroeder TJ, Weiskittel P, Myre SA, Alexander JW, et al. Concomitant administration of cyclosporin and ketoconazole in renal transplant recipients. Lancet 2: 1198–1201, 1989
Fischer G, Wittmann-Liebold B, Lang K, Kiefhaber T, Schmid FX. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 337: 476–478, 1989
Flechner SM, Katz AR, Rogers AJ, Van Buren C, Kahan BD. The presence of cyclosporine in body tissues and fluids during pregnancy. American Journal of Kidney Diseases 5: 60–63, 1985
Flechner SM, Kolbeinsson ME, Tam J, Lum B. The impact of body weight on cyclosporine pharmacokinetics in renal transplant recipients. Transplantation 47: 806–810, 1989
Follath F, Wenk M, Vozeh S, Thiel G, Brunner F, et al. Intravenous cyclosporine kinetics in renal failure. Clinical Pharmacology and Therapeutics 34: 638–643, 1983
Foxwell BMJ, Frazer G, Winters M, Hiestand P, Wenger R, et al. Identification of cyclophilin as the erythrocyte ciclosporin-binding protein. Biochimica et Biophysica Acta 938: 447–455, 1988
Foxwell BMJ, Mackie A, Ling V, Ryffel B. Identification of the multidrug-resistance related P-glycoprotein as a cyclosporine binding protein. Molecular Pharmacology 36: 543–546, 1989
Freeman DJ, Laupacis A, Keown PA, Stiller CR, Carruthers SG. Evaluation of cyclosporin-phenytoin interaction with observations on cyclosporin metabolites. British Journal of Clinical Pharmacology 18: 887–893, 1984
Frey BM, Appenzeller M, Gautschi K, Keller B, Vadas L, et al. Measurements of cyclosporine A by RIA in different centers are not comparable. Transplantation Proceedings 19: 1713–1714, 1987
Frey FJ, Horber FF, Frey BM. Trough levels and concentration time curves of cyclosporine in patients undergoing renal transplantation. Clinical Pharmacology and Therapeutics 43: 55–62, 1988
Gregory CR, Hietala SK, Pedersen NC, Gregory TA, Floyd-Hawkins KA, et al. Cyclosporine pharmacokinetics in cats following topical ocular administration. Transplantation 47: 516–519, 1989
Grevel J. Absorption of cyclosporine A after oral dosing. Transplantation Proceedings 18 (Suppl. 5): 9–15, 1986
Grevel J. Significance of cyclosporine pharmacokinetics. Transplantation Proceedings 20 (Suppl. 2): 428–434, 1988
Grevel J, Kahan BD. Area under the curve monitoring of cyclosporine therapy: the early posttransplant period. Therapeutic Drug Monitoring 13: 89–95, 1991a
Grevel J, Kahan BD. Abbreviated kinetic profiles in area-under-the-curve monitoring of cyclosporine therapy. Clinical Chemistry 37: 1905–1908, 1991b
Grevel J, Nüesch E, Abisch E, Kutz K. Pharmacokinetics of oral cyclosporin A (Sandimmun) in healthy subjects. European Journal of Clinical Pharmacology 31: 211–216, 1986
Grevel J, Reynolds KL, Rutzky LP, Kahan BD. Influence of demographic factors on cyclosporine pharmacokinetics in adult uremic patients. Journal of Clinical Pharmacology 29: 261–266, 1989
Gupta SK, Bakran A, Johnson RWG, Rowland M. Erythromycin enhances the absorption of cyclosporin. British Journal of Clinical Pharmacology 25: 401–402, 1988
Gupta SK, Benet LZ. High-fat meals increase the clearance of cyclosporine. Pharmaceutical Research 7: 46–48, 1990
Gupta SK, Manfro RC, Tomlanovich SJ, Gambertoglio JG, Garovoy MR, et al. Effect of food on the pharmacokinetics of cyclosporine in healthy subjects following oral and intravenous administration. Journal of Clinical Pharmacology 30: 643–653, 1990
Harfmann P, Dittmer R, Busch R, Tenschert W, Arndt R. Cyclosporin A-induced side effects in renal transplantation are related to the ratio of nonspecific/specific cyclosporine blood trough levels as analysed by radioimmunoassay. Transplantation Proceedings 22: 2369–2372, 1990
Hartman NR, Trimble LA, Vederas JC, Jardine I. An acid metabolite of cyclosporine. Biochimica et Biophysica Acta 133: 964–971, 1985
Hashem H, Venkataramanan R, Burckart GJ, Makowka L, Starzl TE, et al. Identification of the aldehydic metabolites. Transplantation Proceedings 20 (Suppl. 1): 176–178, 1988
Henricsson S. A sulfate conjugate of cyclosporin. Pharmacology and Toxicology 66: 53–55, 1990
Holt DW, Marsden JT, Johnston A, Bewick M, Taube DH. Blood cyclosporin concentrations and renal allograft dysfunction. British Medical Journal 293: 1057–1059, 1986
Irish AB, Simons LA, Savdie E, Hayes JM, Simons J. Metabolic changes in renal transplant patients managed with and without cyclosporin. Clinical Transplantation 6: 403–406, 1992
Johnston A, Holt DW. Cyclosporin radioimmunoassay and cardiac transplantation. Lancet 2: 459, 1988
Johnston A, Marsden JT, Hla KK, Henry J, Holt DW. The effect of vehicle on the oral absorption of cyclosporin. British Journal of Clinical Pharmacology 21: 114P, 1986
Kahan BD. Individualization of cyclosporine therapy using pharmacokinetic and pharmacodynamic parameters. Transplantation 40: 457–476, 1985
Kahan BD, Kramer WG, Wideman C, Flechner SM, Lorber MI, van Buren CT. Demographic factors affecting the pharmacokinetics of cyclosporine estimated by radioimmunoassay. Transplantation 41: 459–464, 1986
Kahan BD, Grevel J. Optimization of cyclosporine therapy in renal transplantation by a pharmacokinetic strategy. Transplantation 46: 631–644, 1988
Kahan BD, Ried M, Newburger J. Pharmacokinetics of cyclosporine in human renal transplantation. Transplantation Proceedings 15: 446–453, 1983
Kahan BD, Shaw LM, Holt D, Grevel J, Johnston A. Consensus Document: Hawk’s Cay meeting on therapeutic drug monitoring of cyclosporine. Clinical Chemistry 36: 1510–1516, 1990
Kahan BD, Welsh M, Rutzky L, Lewis R, Knight R, Katz S, et al. The ability of pretransplant test-dose pharmacokinetic profiles to reduce early adverse events after renal transplantation. Transplantation 53: 345–351, 1992
Karlsson MO, Lindberg-Freijs A. Comparison of methods to calculate cyclosporine A bioavailability from consecutive oral and intravenous doses. Journal of Pharmacokinetics and Biopharmaceutics 18: 293–311, 1990
Kasiske BL, Awni WM, Heim-Duthoy KL, Rose M, Rao VK, et al. Alterations in cyclosporine pharmacokinetics after renal transplantation are linked to rapid increases in hematocrit, lipoproteins, and serum protein. Transplantation Proceedings 20 (Suppl. 2): 485–486, 1988
Keown PA, Stiller CR, Laupacis AL, Howson W, Coles R, et al. The effects and side effects of cyclosporine: relationship to drug pharmacokinetics. Transplantation Proceedings 14: 659–661, 1982
Keown PA, Stiller CR, Sinclair NR, Carruthers G, Howson W, et al. The clinical relevance of cyclosporine blood levels as measured by radioimmunoassay. Transplantation Proceedings 15 (Suppl. 1/2): 2438–2441, 1983
Keown PA, Stiller CR, Stawecki M, Freeman D. Pharmacokinetics of cyclosporine in solid organ transplantation. Transplantation Proceedings 18 (Suppl. 5): 160–164, 1986
Keown PA, Stiller CR, Ulan RA, Sinclair NR, Wall WJ, et al. Immunological and pharmacological monitoring in the clinical use of cyclosporin A. Lancet 1: 686–689, 1981
Kessler M, Louis J, Renoult E, Vigneron B, Netter P. Interaction between cyclosporin and erythromycin in a kidney transplant patient. European Journal of Clinical Pharmacology 30: 633–634, 1986
Kivistö KT. A review of assay methods for cyclosporin: clinical implications. Clinical Pharmacokinetics 23: 173–190, 1992
Kolars JC, Awni WM, Merion RM, Watkins PB. First-pass metabolism of cyclosporin by the gut. Lancet 338: 1488–1490, 1991
Koletsky AJ, Harding MW, Handschuhmacher RE. Cyclophilin: distribution and variant properties in normal and neoplastic tissues. Journal of Immunology 137: 1054–1059, 1986
Kronbach T, Fischer V, Meyer UA. Cyclosporine metabolism in human liver: identification of a cytochrome P-450III gene family as the major cyclosporine-metabolizing enzyme explains interactions of cyclosporine with other drugs. Clinical Pharmacology and Therapeutics 43: 630–635, 1988
Krüger HU, Bross-Bach U, Proksch B, Schmidt H, Dopfer R, et al. A case of accidental cyclosporin overdose with pharmacokinetic analysis. Bone Marrow Transplant 3: 167–169, 1988
Kutz K, Nüesch E, Abisch E, Grevel J. Sandimmun: a study on the relative bioavailability of Sandimmun in normal volunteers (soft gelatine capsules versus an oral solution), Document Sandoz Ltd, March 21, 1985
Lake KD. Management of drug interactions with cyclosporine. Pharmacotherapy 11: 110S–118S, 1991
Legg B, Rowland M. Cyclosporin: measurement of fraction unbound in plasma. Journal of Pharmacy and Pharmacology 39: 599–603, 1987
Legg B, Rowland M. Saturable binding of Cyclosporin A to erythrocytes: estimation of binding parameters in renal transplant patients and implications for bioavailability assessment. Pharmaceutical Research 5: 80–85, 1988
LeGrue SJ, Friedman AW, Kahan BD. Binding of cyclosporine by human lymphocytes and phospholipid vesicles. Journal of Immunology 131: 712–718, 1983
Lemaire M, Tillement JP. Role of lipoproteins and erythrocytes in the in vitro binding and distribution of cyclosporin A in the blood. Journal of Pharmacy and Pharmacology 34: 715–718, 1982
Lensmeyer GL, Wieb DA, Carlson IA. Distribution of cyclosporine A metabolites among plasma and cells in whole blood: effect of temperature, hematocrit and metabolite concentration. Clinical Chemistry 35: 56–63, 1989
Lensmeyer GL, Wiebe DA, Carlson IH, Subramanian R. Concentrations of cyclosporin A and its metabolites in human tissues postmortem. Journal of Analytical Toxicology 15: 110–115, 1991
Lindberg A, Odlind B, Tufveson G, Lindström B, Gabrielsson J. The pharmacokinetics of cyclosporine A in uremic patients. Transplantation Proceedings 18 (Suppl. 5): 144–152, 1986
Lindholm A. Therapeutic monitoring of cyclosporin — an update. European Journal of Clinical Pharmacology 41: 273–283, 1991
Lindholm A, Henricsson S, Dahlqvist R. The effect of food and bile acid administration on the relative bioavailability of cyclosporin. British Journal of Clinical Pharmacology 29: 541–548, 1990
Lindholm A, Henricsson S, Lind M, Dahlqvist R. Intraindividual variability in the relative systemic availability of cyclosporin after oral dosing. European Journal of Clinical Pharmacology 34: 461–464, 1988
Lithell H, Odlind B, Selinus I, Lindberg A, Lindström B, et al. Is the plasma lipoprotein pattern of importance for treatment with cyclosporine? Transplantation Proceedings 18: 50–51, 1986
Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66: 807–815, 1991
Loertscher R. Cyclosporine-associated nephrotoxicity is not intractable. Transplantation Proceedings 19: 3486–3489, 1987
Lorenz RG, Garrett N, Turk JW, Scott MG. Problems with therapeutic monitoring of cyclosporine using silicone central venous line samples. Transplantation 52: 1109–1110, 1991
Lucey MR, Kolars JC, Merion RM, Campbell DA, Aldrich M, et al. Cyclosporin toxicity at therapeutic blood levels and cyctochrome P-540 IIIA. Lancet 335: 11–15, 1990
Luke DR, Kasiske BL, Matzke GR, Awni WM, Keane WF. Effects of cyclosporine on the isolated perfused rat kidney. Transplantation 43: 795–799, 1987
Mason J. Renal-side effects of Cyclosporin A. British Journal of Dermatology 122: 71–77, 1990
Maurer G. Metabolism of cyclosporine. Transplantation Proceedings 17 (Suppl. 1): 19–26, 1985
Maurer G, Lemaire M. Biotransformation and distribution in blood of cyclosporine and its metabolites. Transplantation Proceedings 18 (Suppl. 5): 25–34, 1986
Maurer G, Loosli HR, Schreier E, Keller B. Disposition of cyclosporine in several animal species and man: I. Structural elucidation of its metabolites. Drug Metabolism and Disposition 12: 120–126, 1984
McDonald ML, Ardito T, Marks WH, Kashgarian M, Lorber MI. The effect of cyclosporine administration on the cellular distribution and content of cyclophilin. Transplantation 53: 460–466, 1992
Mehta MU, Venkataramanan R, Burckart GJ, Ptachcinski RJ, Delamos B, et al. Effect of bile on cyclosporin absorption in liver transplant patients. British Journal of Clinical Pharmacology 25: 579–584, 1988
Mihatsch MJ, Thiel G, Ryffel B. Hazards of cyclosporine A therapy and recommendations for its use. Journal of Autoimmunity 1: 533–543, 1988
Morse GD, Holdsworth MT, Venuto RC, Gerbasi J, Walshe JJ. Pharmacokinetics and clinical tolerance of intravenous and oral cyclosporine in the immediate postoperative period. Clinical Pharmacology and Therapeutics 44: 654–664, 1988
Moyer TP, Post GR, Sterioff S, Anderson CF. Cyclosporine nephrotoxity is minimized by adjusting dosage on the basis of drug concentration in blood. Mayo Clinic Proceedings 63: 241–247, 1988
Odlind B, Lindberg A, Tufveson G, Lindström B, Froedin L, et al. Longitudinal study of the pharmacokinetics of cyclosporine before and after renal transplantation. Transplantation Proceedings 18: 47–49, 1986
Oldhafer KJ, Schumann G, Wonigeit K, Oellerich M, Ringe B, et al. Cyclosporine A monitoring by radioimmunoassay (RIA) and high-performance liquid chromatography (HPLC) after liver transplantation: influence of route of administration and of liver function on the RIA: HPLC ratio. Transplantation Proceedings 20 (Suppl. 3): 361–365, 1988
Pardridge WM, Triguero D, Yang J, Cancilla PA. Comparison of in vitro and in vivo models of drug transcytosis through the blood-brain barrier. Journal of Pharmacology and Experimental Therapeutics 253: 884–891, 1990
Perico N, Zoja C, Benigni A, Ghilardi F, Gualandris L, et al. Effect of shortterm cyclosporine administration in rats on reninangiotensin and thromboxane A2: possible relevance to the reduction in glomerular filtration rate. Journal of Pharmacology and Experimental Therapeutics 239: 229–235, 1986
Petcher TJ, Weber HP, Rüegger A. Crystal and molecular structure of an iodo derivative of the cyclic undecapeptide cyclosporin A. Helvetica Chimica Acta 59: 1480–1488, 1976
Phillips TM, Karmi SA, Frantz SC, Henriques HF. Absorption profiles of renal allograft recipients receiving oral doses of cyclosporine: a pharmacokinetic study. Transplantation Proceedings 20: 457–461, 1988
Ptachcinski RJ, Burckart GJ, Rosenthal JT, Venkataramanan R, Howrie DL, et al. Cyclosporine pharmacokinetics in children following cadaveric renal transplantation. Transplantation Proceedings 18: 766–767, 1986b
Ptachcinski RJ, Venkataramanan R, Burckart GJ. Clinical pharmacokinetics of cyclosporin. Clinical Pharmacokinetics 11: 107–132, 1986a
Ptachcinski RJ, Venkataramanan R, Burckart GJ, Gray JA, van Thiel DH, et al. Cyclosporine kinetics in healthy volunteers. Journal of Clinical Pharmacology 27: 243–248, 1987
Ptachcinski RJ, Venkataramanan R, Rosenthal JT, Burckart GJ, Taylor RJ, et al. The effect of food on cyclosporine absorption. Transplantation 40: 174–176, 1985
Reymond JP, Steimer JL, Niederberger W. On the dose dependency of cyclosporin A absorption and disposition in healthy volunteers. Journal of Pharmacokinetics and Biopharmaceutics 16: 331–353, 1988
Reynolds KL, Grevel J, Gibbons SY, Welsh MS, Rutzky LP, et al. Cyclosporine pharmacokinetics in uremic patients: influence of different assay methods. Transplantation Proceedings 20 (Suppl. 2): 462–465, 1988
Ried M, Gibbons S, Kwok D, van Buren CT, Flechner S, et al. Cyclosporine levels in human tissues of patients treated for one week to one year. Transplantation Proceedings 15 (Suppl. 1/2): 2434–2437, 1983
Robinson WT, Schran HF, Barry EP. Methods to measure cyclosporine levels-high pressure liquid chromatography, radioimmunoassay and correlation. Transplantation Proceedings 15 (Suppl. 1/2): 2403–2408, 1983
Rosano TG. Effect of hematocrit on cyclosporine (cyclosporin A) in whole blood and plasma of renal-transplant patients, Clinical Chemistry 31: 410–412, 1985
Rosano TG, Freed M, Pell MA, Lempert N. Cyclosporine metabolites in human blood and renal tissue. Transplantation Proceedings 18 (Suppl. 5): 35–40, 1986
Rosano TG, Pell MA, Freed BM, Dybas MT, Lempert N. Cyclosporine and metabolites in blood from renal allograft recipients with nephrotoxicity, rejection, or good renal function: comparative HPLC and monoclonal radioimmunoassay studies. Transplantation Proceedings 20 (Suppl. 2): 330–338, 1988
Rosenthaler J, Keller HP. Comment on cyclosporine assay techniques: an attempt for recommendations. Transplantation Proceedings 22: 1160–1165, 1990
Rowland M, Gupta SK. Cyclosporin-phenytoin interaction: Reevaluation using metabolite data. British Journal of Clinical Pharmacology 24: 329–334, 1987
Rüegger A, Kuhn M, Lichti H, Loosli HR, Huguenin R, et al. Cyclosporine A, ein immunsuppressiv wirksamer Peptidmetabolit aus Trichoderma polysporum (Link ex. Pers.) Rifai. Helvetica Chimica Acta 59: 1075–1092, 1976
Ryffel B, Woerly G, Greiner B, Haendler B, Mihatsch MJ, et al. Distribution of the cyclosporine binding protein cyclophilin in human tissues. Immunology 72: 399–404, 1991
Sandoz AG. Instructions to the Sandimmun Kit, Basel, 1988
Schaefer EJ, Levy RI. Pathogenesis and management of lipoprotein disorders. New England Journal of Medicine 312: 1300–1310, 1985
Schorn T, Kliem V, Bojanovski D, Repp H, Frei U, et al. Long-term immunosuppressive therapy and lipid abnormalities in renal transplant recipients. Nephrology Dialysis Transplantation 3: 581, 1988
Schumacher A, Nordheim A. Progress towards a molecular understanding of cyclosporin A-mediated immunosuppression. Clinical Investigator 70: 773–779, 1992
Schwinghammer TL, Przepiorka D, Venkataramanan R, Wang CP, Burckart GJ, et al. The kinetics of cyclosporine and its metabolites in bone marrow transplant patients. British Journal of Clinical Pharmacology 32: 323–328, 1991
Scott JP, Higenbottam TW. Adverse reactions and interactions of Cyclosporin. Medical Toxicology 3: 107–127, 1988
Sgoutas D, Macmahon W, Love A, Jerkunica I. Interaction of cyclosporin A with human lipoproteins. Journal of Pharmacy and Pharmacology 38: 583–588, 1986
Shaw LM, Bowers L, Demers L, Freeman D, Moyer T, et al. Critical issues in cyclosporin monitoring: report of the task force on cyclosporine monitoring. Clinical Chemistry 33: 1269–1288, 1987
Speck RF, Frey FJ, Frey BM. Cyclosporine kinetics in renal transplant patients as assessed by high-performance liquid chromatography and radioimmunoassay using monoclonal and polyclonal antibodies, Transplantation 47: 802–806, 1989
Starzl TE, Weil R, Iwatsuki S, Klintmalm G, Schroeter GPJ, et al. The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surgery Gynecology and Obstetrics 151: 17–26, 1980
Subbarao MN, Swanson JR, Mueggler PA. Cyclosporine determinations in heart and kidney transplant patients: comparison of high-performance liquid chromatography, polyclonal, and monoclonal methods. Therapeutic Drug Monitoring 11: 53–56, 1989
Takada K, Furuya Y, Yoshikawa H, Muranishi S. Biological and pharmaceutical factors affecting the absorption and lymphatic delivery of ciclosporin A from gastrointestinal tract. Journal of Pharmacobio-Dynamics 11: 80–87, 1988
Tarr BD, Yalkowsky SH. Enhanced intestinal absorption of cyclosporine in rats through the reduction of emulsion droplet size. Pharmaceutical Research 6: 40–43, 1989
Thiel G, Hermle M, Brunner FP. Acutely impaired renal function during intravenous administration of cyclosporine A: a cremophore side-effect. Clinical Nephrology 25 (Suppl. 1): S40–S42, 1986
Thompson ME, Shapiro AP, Johnsen AM, Itzkoff JM, Hardesty RL, et al. The contrasting effects of cyclosporin A and azathioprine on arterial blood pressure and renal function following cardiac transplantation. International Journal of Cardiology 11: 219–229, 1986
Tjia JF, Webber IR, Back DJ. Cyclosporin metabolism by the gastrointestinal mucosa. British Journal of Clinical Pharmacology 31: 344–346, 1991
Ueda CT, Lemaire M, Gsell G, Nussbaumer K. Intestinal lymphatic absorption of cyclosporin A following oral administration in an olive oil solution in rats. Biopharmaceutics and Drug Disposition 4: 113–124, 1983a
Ueda CT, Lemaire M, Misslin P. Pharmacokinetic evaluation of the blood-to-lymph transfer of cyclosporin A in rats. Biopharmaceutics and Drug Disposition 4: 83–94, 1983b
Urien S, Zini R, Lemaire M, Tillement JP. Assessment of cyclosporine A interactions with human plasma lipoproteins in vitro and in vivo in the rat. Journal of Pharmacology and Experimental Therapeutics 253: 305–309, 1990
Venkataramanan R, Koneru B, Wang CCP, Burckart GJ, Caritis SN, Starzl TE. Cyclosporine and its metabolites in mother and baby. Transplantation 46: 468–469, 1988b
Venkataramanan R, Ptachcinski RJ, Burckart GJ, Yang SL, Starzl TE, et al. The clearance of cyclosporine by hemodialysis. Journal of Clinical Pharmacology 24: 528–531, 1984
Venkataramanan R, Wang CP, Habucky K, Ptachcinski RJ, Burckhart GJ, et al. Species-specific cyclosporine metabolism. Transplantation Proceedings 20 (Suppl. 2): 680–683, 1988a
Venkataramanan R, Yang S, Burckart GJ, Ptachcinski RJ, Van Thiel DH, et al. Diurnal variation in cyclosporine kinetics. Therapeutic Drug Monitoring 8: 380–381, 1986
Vine W, Bowers LD. Cyclosporine: structure, pharmacokinetics and therapeutic drug monitoring. Critical Reviews in Clinical Laboratory Sciences 25: 275–311, 1987
Vine W, Bowers LD. Cyclosporine: assay by HPLC and assay with monoclonal antibodies equivalent? Clinical Chemistry 34: 998, 1988
von Wartburg A, Traber R. Chemistry of the natural cyclosporine metabolites. In Borel JF (Ed.) Progress in allergy, Vol. 38, pp. 28–45, Karger, Basel, 1986
Wadhwa NK, Schroeder TJ, O’Flaherty E, Pesce AJ, Myre SA, et al. The effect of oral metoclopramide on the absorption of cyclosporine. Transplantation 43: 211–213, 1987
Wallemacq PE, Lesne M, Otte JB. Cyclosporine monitoring by RIA and HPLC in liver transplantation: clinical correlation. Clinical Transplantation 1: 132–137, 1987
Wallemacq PE, Lhoest G, Dumont P. Isolation, purification and structure elucidation of cyclosporin A metabolites in rabbit and man. Biomedical and Environmental Mass Spectrometry 18: 48–56, 1989
Wang CP, Burckart GJ, Venkataramanan R, Ptachcinski RJ, Cuellar RE, et al. Cyclosporine metabolite profiles in the blood of liver transplant patients. Transplantation Proceedings 20 (Suppl. 1): 173–175, 1988
Wang PP, Simpson E, Meucci V, Morrison M, Lunetta S, et al. Cyclosporine monitoring by fluorescence polarization immunoassay. Clinical Biochemistry 24: 55–58, 1991
Wassef R, Cohen Z, Langer B. Pharmacokinetic profiles of cyclosporine in rats: influence of route and dose. Transplantation 40: 489–493, 1985
Weiser J, Matha V. The insecticidal activity of cyclosporines on mosquito larvae. Journal of Invertebrate Pathology 51: 92–93, 1988
Wenger RM. Structures of cyclosporine and its metabolites. Transplantation Proceedings 22: 1104–1108, 1990
Wenk M, Follath F, Abisch E. Temperature dependency of apparent cyclosporine A concentrations in plasma. Clinical Chemistry 29: 1865, 1983
Wideman CA. Pharmacokinetic monitoring of cyclosporine, Transplantation Proceedings 15 (Suppl. 1/2): 3168–3175, 1983
Williams GM, Irwin B, Burdick J, Pennington L. Intravenous cyclosporine and kidney function: the Johns Hopkins experience. Transplantation Proceedings 18 (Suppl. 1): 66–68, 1986
Wilms HWF, Straeten V, Lison AE. Different pharmacokinetics of cyclosporine A early and late after renal transplantation. Transplantation Proceedings 20 (Suppl. 2): 481–484, 1988
Yee GC, Lennon TP, Gmur DJ, Kennedy MS, Deeg HJ. Age-dependent cyclosporine: pharmacokinetics in marrow transplant recipients. Clinical Pharmacology and Therapeutics 40: 438–443, 1986a
Yee GC, Kennedy MS, Self SG, Storb R, Deeg HJ. Pharmacodynamics of cyclosporine in patients undergoing bone marrow transplantation. Transplantation Proceedings 18: 774–776, 1986b
Yee GC, Lennon TP, Gmur DJ, Kennedy MS, Deeg HJ. Effect of age on cyclosporine pharmacokinetics in marrow transplant recipients. Transplantation Proceedings 19: 1704–1705, 1987
Yee GC, Lennon TP, Gmur DJ, Cheney CL, Oeser D, et al. Effect of obesity on cyclosporine disposition. Transplantation 45: 649–651, 1988a
Yee GC, McGuire TR, Gmur DJ, Lennon TP, Deeg HJ. Blood cyclosporine pharmacokinetics in patients undergoing marrow transplantation: influence of age, obesity, and hematocrit. Transplantation 46: 399–402, 1988b
Yee GC, McGuire TR. Pharmacokinetic drug interactions with cyclosporin. Clinical Pharmacokinetics 19: 319–332 and 400-415, 1990
Ziegler K, Frimmer M, Koepsell H. Photoaffinity labeling of membrane proteins from rat liver and pig kidney with cyclosporine diazirine: involvement of binding to plasma membranes cytotoxic effects. Transplantation 46: 15S–20S, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fahr, A. Cyclosporin Clinical Pharmacokinetics. Clin. Pharmacokinet. 24, 472–495 (1993). https://doi.org/10.2165/00003088-199324060-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199324060-00004