Abstract
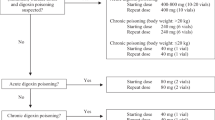
Digitalis glycoside poisoning is an important clinical problem and the development of digoxin-specific antibody fragments (Fab) 30 years ago has changed clinical practice. Nevertheless, doubts still exist as to the appropriate dose indications for therapy. This paper reviews relevant literature, describes the difficulties associated with current treatment protocols and proposes an approach to therapy, which is based on theoretical principles and evidence gleaned from currently available clinical data sets. In patients with ‘acute’ poisoning, serum digoxin concentrations do not equate to the total body burden, as tissue distribution will not have occurred, and the calculations for present protocols, which use serum concentrations, are therefore likely to result in too much antibody being administered. Since a therapeutic quantity of digoxin will have little effect in a normal individual, complete neutralisation of all digoxin is also unnecessary. The pharmacokinetic and dynamic logic of using a smaller initial loading dose than predicted from total body calculations is rational. It is recommended that half the calculated loading dose, either based on serum concentration or history, should be administered and the impact on clinical features observed. If a clinical response is not seen within 1–2 hours, a further similar dose should be given. In the event of a full response, patients should be monitored for 6–12 hours; a second dose should only be given in the event of recurrence of toxicity.
In patients with ‘chronic’ digoxin poisoning, the serum digoxin concentration will reflect the total body load. However, since such patients are invariably receiving digoxin for therapeutic purposes, full neutralisation is again not indicated. In addition, tissue redistribution of digoxin from deeper stores will occur following the binding of biologically active digoxin in the circulation. This process will occur over a number of hours and if the total calculated dose of antibody is administered in a single bolus, significant quantities will be excreted prior to redistribution of digoxin. Pharmacokinetic logic, therefore, suggests that half the calculated loading dose, based on serum concentration, should be administered and the impact on clinical features observed; a second dose should be given in the event of recurrence of toxicity.





Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Withering W. An account of the foxglove and some of its medical uses: with practical remarks on dropsy and other diseases. London: J and J Robinson, 1785
Eddleston M, Rajapakse S, Rajakanthan Jayalath S, et al. Anti-digoxin Fab fragments in cardiotoxicity induced by ingestion of yellow oleander: a randomised controlled trial. Lancet 2000; 355: 967–72
Gowda RM, Cohen RA, Khan IA. Toad venom poisoning: resemblance to digoxin toxicity and therapeutic implications. Heart 2003; 89: e14
Dollery C, editor. Therapeutic drugs. 2nd ed. London: Churchill Livingstone, 1998: D123–D130
Smith TW, Haber E, Yeatman L, et al. Reversal of advanced digoxin intoxication with Fab fragments of digoxin-specific antibodies. N Engl J Med 1976; 294: 797–800
Antman EM, Wenger TL, Butler VP, et al. Treatment of 150 cases of life-threatening digitalis intoxication with digoxin-specific Fab antibody fragments. Circulation 1990; 81: 1744–52
Ujhelyi MR, Robert S. Pharmacological aspects of digoxin-specific Fab therapy in the management of digitalis toxicity. Clin Pharmacokinet 1995; 28: 483–93
Wenger TL, Butler VP, Haber E, et al. Treatment of 63 severely digitalis-toxic patients with digoxin-specific antibody fragments. J Am Coll Cardiol 1985; 5: 118A–23A
Hickey AR, Wenger TL, Carpenter VP, et al. Digoxin immune Fab therapy in the management of digitalis intoxication: safety and efficacy results of an observational surveillance study. J Am Coll Cardiol 1991; 17: 590–8
Woolf AD, Wenger T, Smith TW, et al. The use of digoxin-specific Fab fragments for severe digitalis intoxication in children. N Engl J Med 1992; 326: 1739–44
Smolarz A, Roesch E, Lenz E, et al. Digoxin specific antibody (Fab) fragments in 34 cases of severe digitalis intoxication. J Toxicol Clin Toxicol 1985; 23: 327–40
Taboulet P, Baud FJ, Bismuth C, et al. Acute digitalis intoxication: is pacing still appropriate? J Toxicol Clin Toxicol 1993; 31: 261–73
Schaumann W, Kaufmann B, Neubert P, et al. Kinetics of the Fab fragments of digoxin antibodies and of bound digoxin in patients with severe digoxin intoxication. Eur J Clin Pharmacol 1986; 30: 527–33
Lavaux T, Assemi P, Casset A, et al. Efficiency of a non-equimolar neutralisation of digoxin by immune Fab therapy. J Toxicol Clin Toxicol 2004; 42: 464–5
Acknowledgements
The author gratefully acknowledges discussions with Professor A. Jaeger that contributed to the conclusions presented in this manuscript. No sources of funding were used to assist in the preparation of this review. The author has no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bateman, D.N. Digoxin-Specific Antibody Fragments. Toxicol Rev 23, 135–143 (2004). https://doi.org/10.2165/00139709-200423030-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00139709-200423030-00001