Abstract
▴ A subcutaneously administered, live, high-titre (18 700–60 000 plaque-forming units per dose) varicella zoster virus (VZV) vaccine (zoster vaccine) of the Oka/Merck strain has been evaluated for the prevention of herpes zoster and the reduction of zoster-associated pain in adults aged ≥60 years.
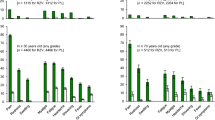
▴ Zoster vaccine, when compared with placebo, reduced the burden of herpes zoster illness by 61%, the incidence of herpes zoster by 51% and the incidence of postherpetic neuralgia by 67% during more than 3 years of surveillance.
▴ The zoster vaccine caused an initial 1.7-fold rise in VZV antibody titre after 6 weeks that declined progressively over 3 years.
▴ Increases in γ-interferon-secreting peripheral blood mononuclear cells were 2.2-fold greater with the zoster vaccine than with placebo 6 weeks after vaccination.
▴ Zoster vaccine was generally well tolerated. The most frequently reported adverse reactions following vaccination were injection-site reactions; the only systemic adverse event with zoster vaccine that differed significantly in incidence from that with placebo was headache.


Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Ampofo K, Saiman L, LaRussa P, et al. Persistence of immunity to live attenuated varicella vaccine in healthy adults. Clin Infect Dis 2002 Mar 15; 34: 774–9
Schmader K. Herpes zoster in older adults. Clin Infect Dis 2001 May 15; 32(10): 1481–6
Jumaan AO, Yu O, Jackson LA, et al. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002. J Infect Dis 2005 Jun 15; 191(12): 2002–7
Douglas MW, Johnson RW, Cunningham AL. Tolerability of treatments for postherpetic neuralgia. Drug Saf 2004; 27(15): 1217–33
Lilie M, Wassilew SW. The role of antivirals in the management of neuropathic pain in the older patient with herpes zoster [published erratum appears in Drugs Aging 2004; 21 (3): 201]. Drugs Aging 2003; 20(8): 561–70
Kost RG, Straus SE. Postherpetic neuralgia: pathogenesis, treatment, and prevention. N Engl J Med 1996 Jul 4; 335(1): 32–42
Whitley RJ, Weiss H, Gnann JW Jr, et al. Acyclovir with and without prednisone for the treatment of herpes zoster: a randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med 1996 Sep 1; 125(5): 376–83
Wood MJ, Johnson RW, McKendrick MW, et al. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med 1994 Mar 31; 330(13): 896–900
Thomas SL, Wheeler JG, Hall AJ. Contacts with varicella or with children and protection against herpes zoster in adults: a case-control study. Lancet 2002 Aug 31; 360(9334): 678–82
Edmunds WJ, Brisson M. The effect of vaccination on the epidemiology of varicella zoster virus. J Infect 2002 May; 44(4): 211–9
Goldman GS. Cost-benefit analysis of universal varicella vaccination in the U.S. taking into account the closely related herpes-zoster epidemiology. Vaccine 2005 May 9; 23(25): 3349–55
Merck & Co. Inc. FDA approves ZOSTAVAX®, Merck’s new vaccine for prevention of shingles in adults age 60 and older [media release]. 2006
Levin MJ, Murray M, Rotbart HA, et al. Immune response of elderly individuals to a live attenuated varicella vaccine. J Infect Dis 1992 Aug; 166: 253–9
Levin MJ, Murray M, Zerbe GO, et al. Immune responses of elderly persons 4 years after receiving a live attenuated varicella vaccine. J Infect Dis 1994 Sep; 170: 522–6
Levin MJ, Barber D, Goldblatt E, et al. Use of a live attenuated varicella vaccine to boost varicella-specific immune responses in seropositive people 55 years of age and older: duration of booster effect. J Infect Dis 1998 Nov; 178Suppl. 1: 109–12
Levin MJ, Ellison MC, Zerbe GO, et al. Comparison of a live attenuated and an inactivated varicella vaccine to boost the varicella-specific immune response in seropositive people 55 years of age and older. Vaccine 2000; 18(25): 2915–20
Levin MJ, Smith JG, Kaufhold RM, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis 2003 Nov 1; 188(9): 1336–44
Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005 Jun 2; 352(22): 2271–84 plus supplementary material [online]. Available from URL: http://content.nejm.org/cgi/content/full/352/22/2271/DC1 [Accessed 2006 Jul 21]
Rohan P. FDA clinical briefing document for Merck & Co., Inc. Zoster vaccine live (Oka/Merck) Zostavax™ [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/05/briefing/5-4198B2_1.pdf [Accessed 2005 Dec 20]
Merck & Co. Ltd. Zostavax™ (Zoster Vaccine Live [Oka/Merck]) advisory committee meeting background document [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/05/briefing/5-4198B2_3.pdf [Accessed 2005 Dec 20]
Merck & Co. Inc. Varivax® [varicella virus vaccine live (Oka/Merck)] [online]. Available from URL: http://www.merck.com/product/usa/pi_circulars/v/varivax/varivax_pi.pdf [Accessed 2006 Jan 9]
Merck & Co. Inc. ZOSTAVAX® [zoster vaccine live (Oka/Merck)] [online]. Available from URL: http://www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi.pdf [Accessed 2006 May 30]
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robinson, D.M., Perry, C.M. Zoster Vaccine Live (Oka/Merck). Drugs Aging 23, 525–531 (2006). https://doi.org/10.2165/00002512-200623060-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002512-200623060-00007