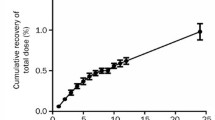
Summary
The volumes of distribution of many acidic drugs have been shown to be close to that of their binding protein, i.e. serum albumin. The distribution of basic drugs mainly bound to α1-acid glycoprotein (AAG) can be questioned with respect to its dependency upon the distribution of this plasma protein. So, a pharmacokinetic study was performed in 7 subjects with human 125I-labelled α1-acid glycoprotein. The steady-state volume of distribution was found to be 5.37 ± 0.82L. The central volume was 3.23 ± 0.33L, close to that of plasma volume and the peripheral volume was 2.14 ± 0.63L. These data allowed the establishment of an equation giving access to the volume of distribution of a basic drug by relating its unbound fraction to physiological distribution of α1-acid glycoprotein. The values yielded by this equation show that the actual and calculated volumes of distribution of basic drugs mainly bound to AAG are discrepant. This protein is thus not the main factor controlling the distribution of basic drugs within the body.
Similar content being viewed by others
References
Austin KL, Mather LE, Philpot CR, McDonald PJ. Intersubject and dose-related variability after intravenous administration of erythromycin. British Journal of Clinical Pharmacology 10: 273–279, 1980
Belcher EH, Berlin NI, Dudley RA, Garby L, Heimpel H, et al. Recommended methods for measurement of red-cell and plasma volume. Journal of Nuclear Medicine 21: 793–800, 1980
Belpaire FM, Bogaert MG, Rosseneu M. Binding of β-adrenoceptor blocking drugs to human serum albumin, to α-acid glycoprotein and to human serum. European Journal of Clinical Pharmacology 22: 253–256, 1982
Benet LZ, Sheiner LZ. Design and optimization of dosage regimens; Pharmacokinetic data. In Goodman et al. (Eds) The pharmacological basis of therapeutics, pp. 1675–1737, MacMillan New York, 1980
Berson SA, Yalow RS. Distribution and metabolism of I131 labeled protein in man. Federation Proceedings 16: 135–185, 1957
Berson SA, Yalow RS, Schreiber SS, Post J. Tracer experiments with I131 labeled human serum albumin: distribution and degradation studies. Journal of Clinical Investigation 32: 746–768, 1953
D’Athis Ph, Richard MO, De Lauture D, Rey E, Bouvier-D’Yvoire M, et al. Étude comparative des disponibilités de deux formes de spironolactone: application à la rationalisation des posologies. Thérapie 36: 443–449, 1981
Hunter WM, Greenwood FC. Preparation of iodine 131I labeled human growth hormone of high specific activity. Nature 184: 495–496, 1962
Lemaire M, Urien S, Albengres E, Riant P, Zini R, et al. Lipoprotein binding of drugs. In Reidenberg M & Erill F (Eds) Proceedings of the Symposium on Drug Protein Binding, Mallorca, 1984, Praeger, New York, in press, 1986
Lima JJ, Boudoulas H, Blanford M. Concentration-dependence of disopyramide binding to plasma protein and its influence on kinetics and dynamics. Journal of Pharmacology and Experimental Therapeutics 219: 741–747, 1981
Mason WD, Winer N. Pharmacokinetics of oxprenolol in normal subjects. Clinical Pharmacology and Therapeutics 20: 401–412, 1976
Meffin PG, Robert EW, Winkle RA, Harapat S, Peters FA, et al. Role of concentration dependent plasma protein binding in disopyramide disposition. Journal of Pharmacokinetics and Biopharmaceutics 7: 29–46, 1979
Meresaar U, Nilsson MI, Holmstrand J, Angaad E. Single dose pharmacokinetics and bioavailability of methadone in man studied with a stable isotope method. European Journal of Clinical Pharmacology 202: 473–478, 1981
Oie S, Tozer TN. Effect of altered plasma protein binding on apparent volume of distribution. Journal of Pharmaceutical Sciences 68: 1203–1205, 1979
Paxton JW. Alpha I acid glycoprotein and binding of basic drugs. Methods and Findings in Experimental and Clinical Pharmacology 5: 635–648, 1983
Piafsky KM. Disease induced change in the plasma binding of basic drugs. Clinical Pharmacokinetics 5: 546–562, 1980
Piafsky KM, Borga O. Plasma protein binding of basic drugs. II. Importance of α1-glycoprotein for interindividual variation. Clinical Pharmacology and Therapeutics 22: 545–549, 1977
Prandota J, Tillement JP, D’Athis Ph, Campos H, Barré J. Binding of erythromycin base to human plasma proteins. Journal of International Medicine Research 8 (Suppl. 2): 1–8, 1980
Romach MK, Piafsky KM, Abel JG, Khouw V, Sellers EM. Methadone binding to orosomucoid (∝1acid glycoprotein): determinant of free fraction in plasma. Clinical Pharmacology and Therapeutics 29: 211–217, 1981
Routledge PA, Barchowsky A, Bjornsson TD, Kitchell BB, Shand DG. Lidocaine plasma protein binding. Clinical Pharmacology and Therapeutics 27: 347–351, 1980
Rowland M, Thompson PD, Guichard A, Melmon KL. Disposition kinetics of lidocaine in normal subjects. Annals of the New-York Academy of Sciences 179: 383–398, 1971
Tillement JP. The relationship between plasma protein binding distribution and pharmacokinetics of drugs. In Boissier et al. (Eds) Advances in pharmacology and therapeutics, Vol. 7, pp. 103–111, Pergamon Press, Oxford and New York, 1978
Tillement JP, Zini R, Glasson S, Jacotot B. Fixation plasmatique et pharmacocinétique des médicaments. Revue de Médecine Interne III: 75–80, 1982
Tiula E, Neuvonen PJ. Antiepileptic drug and α1-acid glycoprotein. New England Journal of Medecine 307: 1148, 1982
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brée, F., Houin, G., Barré, J. et al. Pharmacokinetics of Intravenously Administered 125I-Labelled Human α1-Acid Glycoprotein. Clin-Pharmacokinet 11, 336–342 (1986). https://doi.org/10.2165/00003088-198611040-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-198611040-00006