Summary
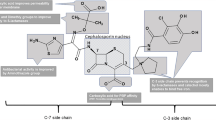
Cefpirome is a new cephalosporin that exhibits similar in vitro potency to ceftazidime against Gram-negative organisms but has significantly greater in vitro potency against Gram-positive organisms. Cefpirome differs from cefotaxime in that a 3′-pyridinium moiety replaces the acetoxy moiety of cefotaxime. This structural change imparts greater β-lactamase stability, increases the ability to penetrate the outer membrane of Gram-negative bacteria, and enhances activity against Gram-positive organisms.
The pharmacokinetic properties of cefpirome are typical of cephalosporins. The drug can be administered by intravenous or intramuscular injection, but is not well absorbed after oral administration. Bioavailability following intramuscular injection exceeds 90%. Cefpirome exhibits low protein binding (≈10%) and has a volume of distribution similar to extracellular fluid volume.
Cefpirome penetrates the prostate gland, lung, blister fluid, cerebrospinal fluid and peritoneal fluid, reaching concentrations that are similar to those achieved by other later generation cephalosporins. Approximately 80% of an intravenous dose is eliminated unchanged in the urine. No active metabolites of cefpirome have been identified. The elimination half-life of cefpirome is approximately 2 hours. Elimination appears to be primarily by glomerular filtration since the total clearance of cefpirome is approximately equal to creatinine clearance.
The time during which drug concentrations exceed the minimum inhibitory concentration (MIC) represents the most clinically important pharmacodynamic parameter for β-lactam agents. When cefpirome is administered at a dosage of 2g every 12 hours to patients without renal insufficiency [creatinine clearance 70 ml/min (4.2 L/h)], drug concentrations continuously remain above the MIC for pathogens with MIC values of ⩽2 µg/ml. With this dosage regimen, drug concentrations will be above the MIC for a pathogen with an MIC of 4 µg/ml for 80% of the dosage interval. The time above MIC for pathogens with an MIC of 8 µg/ml is only 60% of the dosage interval.
Similar content being viewed by others
References
Badian M, Malerczyk V, Collins JD, Dixon GT, Verho M, et al. Safety, tolerance and pharmacokinetics of 2.0g cefpirome (HR 810) after single and multiple dosing. Chemotherapy 34: 367–373, 1988
Baldwin DR, Maxwell SJR, Honeybourne D, Andrews JM, Ashby JP, et al. The penetration of cefpirome into the potential sites of pulmonary infection. Journal of Antimicrobial Chemotherapy 28: 79–86, 1991
Barza M. Principles of tissue penetration of antibiotics. Journal of Antimicrobial Chemotherapy 8 (Suppl. C): 7–28, 1981
Barza M, Cuchural G. The penetration of antibiotics into the prostate in chronic bacterial prostatitis. European Journal of Clinical Microbiology 3: 503–505, 1984
Brown RF, Kinnick MD, Morin JM, Vasileff RT, Counter FT, et al. Synthesis and biologic evaluation of a series of parenteral 3′-quaternary ammonium cephalosporins. Journal of Medicinal Chemistry 33: 2114–2121, 1990
Christ W. Pharmacological properties of cephalosporins. Infection 19 (Suppl. 5): s244–s252, 1991
Craig WA, Ebert SC. Continuous infusion of β-lactam antibiotics. Antimicrobial Agents and Chemotherapy 36: 2577–2583, 1992
Cunha BA. Antibiotic pharmacokinetic considerations in pulmonary infections. Seminars in Respiratory Infections 6: 168–182, 1991
Eng RHK, Cherubin CE, Smith SM, Buccini F, Harris R. In-vitro and in-vivo activity of cefpirome (HR 810) against methicillin-susceptible and -resistant Staphylococcus aureus and Streptococcus faecalis. Journal of Antimicrobial Chemotherapy 23: 373–381, 1989
Fair WR, Cordonnier JJ. The pH of prostatic fluid: a reappraisal and therapeutic implications. Journal of Urology 120: 695–698, 1978
Gargalianos P, Oppenheim BA, Skepastianos P, Livermore DM, Williams RJ. Activity of cefpirome (HR 810) against Pseudomonas aeruginosa strains with characterized resistance mechanisms to β-lactam antibiotics. Journal of Antimicrobial Chemotherapy 22: 841–848, 1988
Goldstein EJC, Citron DM. Comparative in vitro inhibitory and killing activity of cefpirome, ceftazidime, and cefotaxime against Pseudomonas aeruginosa, enterococci, Staphylococcus epidermidis, and methicillin-susceptible and -resistant and tolerant and non-tolerant Staphylococcus aureus. Antimicrobial Agents and Chemotherapy 28: 160–162, 1985
Grötsch H, Hajdú P. Interference by the new antibiotic cefpirome and other cephalosporins in clinical laboratory tests, with special regard to the ‘Joffe’ reaction. Journal of Clinical Chemistry and Clinical Biochemistry 25: 49–52, 1987
Hancock REW, Bellido F. Factors involved in the enhanced efficacy against gram-negative bacteria of fourth generation cephalosporins. Journal of Antimicrobial Chemotherapy 29 (Suppl. A): 1–6, 1992
Jones RN, Pfaller MA, Allen SD, Gerlach EH, Fuchs PC, et al. Antimicrobial activity of cefpirome an update compared to five third-generation cephalosporins against nearly 6000 recent clinical isolates from five medical centers. Diagnostic Microbiology and Infectious Diseases 14: 361–364, 1991
Kavi J, Andrews JM, Ashby JP, Hillman G, Wise R. Pharmacokinetics and tissue penetration of cefpirome, a new cephalosporin. Journal of Antimicrobial Chemotherapy 22: 911–916, 1988
Kavi J, Ashby JP, Wise R, Donovan IA. Intraperitoneal penetration of cefpirome. European Journal of Clinical Microbiology and Infectious Diseases 8: 556–558, 1989
Kearns GL, Johnson VA, Hendry IR, Wells TG. Microanalytical high-performance liquid Chromatographic assay for cefpirome in human milk and urine. Journal of Chromatography 574: 356–360, 1992
Kobayashi S, Arai S, Hayashi S, Fujimoto K. β-lactamase stability of cefpirome (HR-810), a new cephalosporin with a broad antimicrobial spectrum. Antimicrobial Agents and Chemotherapy 30: 713–718, 1986
Lameire N, Malerczyk V, Drees B, Lehr K, Rosenkranz B. Single dose pharmacokinetics of cefpirome in patients with renal impairment. Clinical Pharmacology and Therapeutics 52: 24–30, 1992
Lameire NH, Rosenkranz B, Malerczyk V. Influence of renal functional impairment and haemodialysis on the pharmacokinetics of cefpirome. Programs and abstracts of the Twenty-ninth Interscience Conference on Antimicrobial Agents and Chemotherapy, pp. 308, Houston, September 17–20, 1989
Lassman HB, Hubbard JW, Chen B, Puri SK. Lack of interaction between cefpirome and alcohol. Journal of Antimicrobial Chemotherapy 29 (Suppl. A): 47–50, 1992
Lattrell R, Blumbach J, Duerckheimer W, Fehlhaber H, Fleischmann K, et al. Synthesis and structure-activity relationships in the cefpirome series I. Journal of Antibiotics 41: 1374–1394, 1988
Lattrell R, Durckheimer W, Klesel N, Kirrstetter R, Seibert G, et al. HR 810, a new parenteral cephalosporin. Proceedings of the 13th International Congress of Chemotherapy, pp. 99/1–99/4, Vienna, August 28-September 2, 1983
Maass L, Malerczyk V, Verho M. Pharmacokinetics of cefpirome (HR 810), a new cephalosporin derivative administered intramuscularly and intravenously to healthy volunteers. Infection 15: 207–210, 1987a
Maass L, Malerczyk V, Verho M, Hajdú P, Seeger K, et al. Dose linearity testing of intravenous cefpirome (HR 810), a novel cephalosporin derivate. Infection 15: 202–206, 1987b
Malerczyk V, Maass L, Verho M, Hajdú P, Kiesel N, et al. Single and multiple dose pharmacokinetics of intravenous cefpirome (HR 810), a novel cephalosporin derivative. Infection 15: 211–214, 1987
Meyer BH, Muller FO, Luus HG, Drees B, Röthig HJ, et al. Safety, tolerance and pharmacokinetics of cefpirome administered intramuscularly to healthy subjects. Journal of Antimicrobial Chemotherapy 29 (Suppl. A): 63–70, 1992
Murdoch MB, Peterson LR. Antimicrobial penetration into polymorphonuclear leukocytes and alveolar macrophages. Seminars in Respiratory Infections 6: 112–121, 1991
Nakayama I, Akieda Y, Yamaji E, Nitta Y, Ohishi M, et al. Single-and multiple-dose pharmacokinetics of intravenous cefpirome (HR 810) to healthy volunteers. Journal of Clinical Pharmacology 32: 256–266, 1992
Nikaido H, Liu W, Rosenberg U. Outer membrane permeability and β-lactamase stability of dipolar ionic cephalosporins containing methoxyamino substituents. Antimicrobial Agents and Chemotherapy 34: 337–342, 1990
Nix DE, Goodwin SD, Peloquin CA, Rotella DL, Schentag JJ. Antibiotic tissue penetration and its relevance: models of tissue penetration and their meaning. Antimicrobial Agents and Chemotherapy 35: 1947–1952, 1991a
Nix DE, Goodwin SD, Peloquin CA, Rotella DL, Schentag JJ. Antibiotic tissue penetration and its relevance: impact of tissue penetration on infection response. Antimicrobial Agents and Chemotherapy 35: 1953–1959, 1991b
Nix DE, Wilton JH, Velasquez N, Budny JL, Lassman HB, et al. Cerebrospinal fluid penetration of cefpirome in patients with non-inflamed meninges. Journal of Antimicrobial Chemotherapy 29 (Suppl. A): 51–57, 1992
Paradis D, Vallée F, Allard S, Bisson C, Daviau N, et al. Comparative study of pharmacokinetics and serum bactericidal activities of cefpirome, ceftazidime, ceftriaxone, imipenem, and ciprofloxacin. Antimicrobial Agents and Chemotherapy 36: 2085–2092, 1992
Saxby MF, Arkell DG, Andrews JM, Wise R. Penetration of cefpirome into prostatic tissue. Journal of Antimicrobial Chemotherapy 25: 488–490, 1990
Schäfer V, Shah PM, Doerr HW, Zieman M, Hellwich S, et al. In vitro activity of cefpirome against isolates from patients with urinary tract, lower respiratory tract and wound infections. Journal of Antimicrobial Chemotherapy 29 (Suppl. A): 7–12, 1992
Schentag JJ. Clinical significance of antibiotic tissue penetration. Clinical Pharmacokinetics 16 (Suppl. 1): 25–31, 1989
Sugioka T, Asano T, Chikaraishi Y, Suzuki E, Sano A, et al. Stability and degradation pattern of cefpirome (HR-810) in aqueous solution. Chemical and Pharmaceutical Bulletin 38: 1998–2002, 1990
Tabata S, Shimura T, Maeda T, Hayashi S. Protein binding of cefpirome sulfate in man and animals and its displacing effect on bilirubin bound to human serum albumin. Chemotherapy (Tokyo) 39 (Suppl. 1): 109–114, 1991
Täuber MG, Sande MA. General principles of therapy of pyogenic meningitis. Infectious Disease Clinics of North America 4: 661–676, 1990
Tullus K, Olsson-Liljequist B, Lundström G, Burman LG. Antibiotic susceptibility of 629 bacterial blood and CSF isolates from Swedish infants and therapeutic implications. Acta Paediatrica Scandinavica 80: 205–212, 1991
Turley CP, Kearns GL, Jacobs RF. Microanalytical high-performance liquid chromatography assay for cefpirome (HR 810) in serum. Antimicrobial Agents and Chemotherapy 32: 1481–1483, 1988
Uihlein M, Kiesel N, Seeger K. Determination of cefpirome (HR 810) in serum and urine. Infection 16: 135–140, 1988
Valcke Y, Pauwels R, Van Der Straeten M. Pharmacokinetics of antibiotics in the lungs. European Respiratory Journal 3: 715–722, 1990
Verho M, Maass L, Malerczyk V, Grötsch H. Renal tolerance of cefpirome (HR 810), a new cephalosporin antibiotic. Infection 15: 215–219, 1987
Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, et al. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. Journal of Infectious Diseases 158: 831–847, 1988
Wheat PF, Spencer RC. In vitro activity of cefpirome (HR 810) against Enterococcus species. European Journal of Microbiology and Infectious Diseases 9: 235–237, 1990
Wise R. Methods for evaluating the penetration of β-lactam antibiotics into tissues. Reviews of Infectious Diseases 8 (Suppl. 3): s325–s332, 1986
Wise R, Andrews JM, Cross C, Piddock JV. The antimicrobial activity of cefpirome, a new cephalosporin. Journal of Antimicrobial Chemotherapy 15: 449–456, 1985
Wolff M, Chavanet P, Kazmierczak A, Pechinot A, Dematons C, et al. Diffusion of cefpirome into the cerebrospinal fluid of patients with purulent meningitis. Journal of Antimicrobial Chemotherapy 29 (Suppl. A): 59–62, 1992
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Strenkoski, L.C., Nix, D.E. Cefpirome Clinical Pharmacokinetics. Clin. Pharmacokinet. 25, 263–273 (1993). https://doi.org/10.2165/00003088-199325040-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199325040-00002