Abstract
Although activity of cytochrome P450 isoenzymes (CYPs) plays a major role in the fate of anticancer agents in patients, there are relatively few clinical studies that evaluate drug metabolism with therapeutic outcome. Nevertheless, many clinical reports in various non-oncology fields have shown the dramatic importance of CYP activity in therapeutic efficacy, safety and interindividual variability of drug pharmacokinetics. Moreover, variability of drug metabolism in the liver as well as in cancer cells must also be considered as a potential factor mediating cancer resistance.
This review underlines the role of drug metabolism mediated by CYPs in pharmacokinetic variability, drug resistance and safety. As examples, biotransformation pathways of tamoxifen, paclitaxel and imatinib are reviewed.
This review emphasises the key role of therapeutic drug monitoring as a complementary tool of investigation to in vitro data. For instance, pharmacokinetic data of anticancer agents have not often been published within subpopulations of patients who show ultra-rapid, extensive or poor metabolism (e.g. due to CYP2D6 and CYP2C19 genotypes).
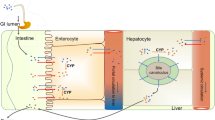
Besides kinetic variability in the systemic circulation, induction of CYP activity may participate in creating drug resistance by speeding up the cancer agent degradation specifically in the target cells. For one cancer agent, various mechanisms of resistance are usually identified within different cell clones. This review also tries to emphasise that drug resistance mediated by CYP activity in cancer cells should be taken into consideration to a greater degree.
The unequivocal identification of the metabolising enzymes involved in clinical conditions will eventually allow improvement and individualisation of anticancer agent therapy, i.e. drug dosage and selection. In addition, a more complete understanding of the metabolism of anticancer agents will assist in the prediction of drug-drug interactions, as anticancer agent combinations are becoming more prevalent.





Similar content being viewed by others
References
Kivisto KT, Kroemer HK, Eichelbaum M. The role of human cytochrome P450 enzymes in the metabolism of anticancer agents: implications for drug interactions. Br J Clin Pharmacol 1995; 40: 523–30
McLeod HL. Clinically relevant drug-drug interactions in oncology. Br J Clin Pharmacol 1998; 45: 539–44
Beijnen JH, Schellens JH. Drug interactions in oncology. Lancet Oncol 2004; 5(8): 489–96
Hon YY, Evans WE. Making TDM work to optimize cancer chemotherapy: a multidisciplinary team approach. Clin Chem 1998; 44: 388–400
Innocenti F, Iyer L, Ratain MJ. Pharmacogenetics: a tool for individualizing antineoplastic therapy. Clin Pharmacokinet 2000; 39: 315–25
Ingelman-Sundberg M, Daly AK, Oscarson M, et al. Human cytochrome P450 (CYP) genes: recommendations for the nomenclature of alleles. Pharmacogenetics 2000; 10: 91–3
Ingelman-Sundberg M, Oscarson M, Daly AK, et al. Human cytochrome P-450 (CYP) genes: a web page for the nomenclature of alleles. Cancer Epidemiol Biomarkers Prev 2001 Dec; 10(12): 1307–8
Iyer L, Ratain MJ. Pharmacogenetics and cancer chemotherapy. Eur J Cancer 1998; 34: 1493–9
MacLeod SL, Nowell S, Massengill J, et al. Cancer therapy and polymorphisms of cytochromes P450. Clin Chem Lab Med 2000; 38: 883–7
Kall MA, Vang O, Clausen J. Effects of dietary broccoli on human drug metabolising activity. Cancer Letters 1997; 114: 169–70
Krishna DR, Klotz U. Extrahepatic metabolism of drugs in humans. Clin Pharmacokinet 1994; 26: 144–60
Yu LJ, Matias J, Scudiero DA, et al. P450 enzyme expression patterns in the NCI human tumor cell line panel. Drug Metab Dispos 2001; 29: 304–12
Doherty MM, Michael M. Tumoral drug metabolism: perspectives and therapeutic implications. Curr Drug Metab 2003; 4: 131–49
Huang L, Wring SA, Woolley JL, et al. Induction of P-glycoprotein and cytochrome P450 3A by HIV protease inhibitors. Drug Metab Dispos 2001; 29: 754–60
Schrenk D, Gant TW, Michalke A, et al. Metabolic activation of 2-acetylaminofluorene is required for induction of multidrug resistance gene expression in rat liver cells. Carcinogenesis 1994; 15: 2541–6
Burt RK, Thorgeirsson SS. Coinduction of MDR-1 multidrugresistance and cytochrome P-450 genes in rat liver by xenobiotics. J Natl Cancer Inst 1988; 80: 1383–6
Kim RB, Wandel C, Leake B, et al. Interrelationship between substrates and inhibitors of human CYP3A and P-glycoprotein. Pharm Res 1999; 16: 408–14
Wacher VJ, Wu CY, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog 1995; 13: 129–34
Cummings J, Zelcer N, Allen JD, et al. Glucuronidation as a mechanism of intrinsic drug resistance in colon cancer cells: contribution of drug transport proteins. Biochem Pharmacol 2004; 67(1): 31–9
Cummings J, Boyd G, Ethell BT, et al. Enhanced clearance of topoisomerase I inhibitors from human colon cancer cells by glucuronidation. Biochem Pharmacol 2002; 63(4): 607–13
Bock KW. Vertebrate UDP-glucuronosyltransferases: functional and evolutionary aspects. Biochem Pharmacol 2003; 66(5): 691–6
Dehal SS, Kupfer D. Evidence that the catechol 3,4-dihydroxytamoxifen is a proximate intermediate to the reactive species binding covalently to proteins. Cancer Res 1996; 56: 1283–90
Dehal SS, Kupfer D. Cytochrome P-450 3A and 2D6 catalyze Ortho hydroxylation of 4-hydroxytamoxifen and 3-hydroxytamoxifen (droloxifene) yielding tamoxifen catechol: involvement of catechols in covalent binding to hepatic proteins. Drug Metab Dispos 1999; 27: 681–8
Pelkonen O, Raunio H. Metabolic activation of toxins: tissuespecific expression and metabolism in target organs. Environ Health Perspect 1997; 105: 767–74
Meyer UA, Zanger UM. Molecular mechanisms of genetic polymorphisms of drug metabolism. Annu Rev Pharmacol Toxicol 1997; 37: 269–96
Aklillu E, Persson I, Bertilsson L, et al. Frequent distribution of rapid metabolizers of debrisoquine in an Ethiopian population carrying duplicated and multiduplicated functional CYP2D6 alleles. J Pharmacol Exp Ther 1996; 278: 441–6
Tribut O, Lessard Y, Reymann JM, et al. Pharmacogenomics. Med Sci Monit 2002; 8: 152–63
Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol 1999; 39: 1–17
Lamba JK, Lin YS, Schuetz EG, et al. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev 2002; 54: 1271–94
Lin YS, Dowling AL, Quigley SD, et al. Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Mol Pharmacol 2002; 62: 162–72
Tateishi T, Watanabe M, Moriya H, et al. No ethnic difference between Caucasian and Japanese hepatic samples in the expression frequency of CYP3A5 and CYP3A7 proteins. Biochem Pharmacol 1999; 57: 935–9
Dai D, Tang J, Rose R, et al. Identification of variants of CYP3A4 and characterization of their abilities to metabolize testosterone and chlorpyrifos. J Pharmacol Exp Ther 2001; 299: 825–31
Hirth J, Watkins PB, Strawderman M, et al. The effect of an individual’s cytochrome CYP3A4 activity on docetaxel clearance. Clin Cancer Res 2000; 6: 1255–8
Tran JQ, Kovacs SJ, McIntosh TS, et al. Morning spot and 24-hour urinary 6 beta-hydroxycortisol to Cortisol ratios: intraindividual variability and correlation under basal conditions and conditions of CYP 3A4 induction. J Clin Pharmacol 1999; 39: 487–94
Yamamoto N, Tamura T, Kamiya Y, et al. Correlation between docetaxel clearance and estimated cytochrome P450 activity by urinary metabolite of exogenous Cortisol. J Clin Oncol 2000; 18: 2301–8
Goh BC, Lee SC, Wang LZ, et al. Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol 2002; 20: 3683–90
Goldstein JA. Clinical relevance of genetic polymorphisms in the human CYP2C subfamily. Br J Clin Pharmacol 2001; 52: 349–55
Dai D, Zeldin DC, Blaisdell JA, et al. Polymorphisms in human CYP2C8 decrease metabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics 2001; 11: 597–607
Murray GI, Taylor MC, McFadyen MC, et al. Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Res 1997; 57: 3026–31
Rochat B, Morsman JM, Murray GI, et al. Human CYP1B1 and anticancer agent metabolism: mechanism for tumor-specific drug inactivation? J Pharmacol Exp Ther 2001; 296: 537–41
McFadyen MC, McLeod HL, Jackson FC, et al. Cytochrome P450 CYP1B1 protein expression: a novel mechanism of anticancer drug resistance. Biochem Pharmacol 2001; 62: 207–12
Chua MS, Kashiyama E, Bradshaw TD, et al. Role of CYP1 A1 in modulation of antitumor properties of the novel agent 2-(4-amino-3-methylphenyl)benzothiazole (DF 203, NSC 674495) in human breast cancer cells. Cancer Res 2000; 60: 5196–203
Dhaini HR, Thomas DG, Giordano TJ, et al. Cytochrome P450 CYP3A4/5 expression as a biomarker of outcome in osteosarcoma. J Clin Oncol 2003; 21: 2481–5
Miyoshi Y, Ando A, Takamura Y, et al. Prediction of response to docetaxel by CYP3A4 mRNA expression in breast cancer tissues. Int J Cancer 2002; 97: 129–32
Cummings J, Ethell BT, Jardine L, et al. Glucuronidation as a mechanism of intrinsic drug resistance in human colon cancer: reversal of resistance by food additives. Cancer Res 2003; 63(23): 8443–50
Kan O, Kingsman S, Naylor S. Cytochrome P450-based cancer gene therapy: current status. Expert Opin Biol Ther 2002; 2: 857–68
Chen L, Waxman DJ. Cytochrome P450 gene-directed enzyme prodrug therapy (GDEPT) for cancer. Curr Pharm Des 2002; 8: 1405–16
Rochat B. Evaluation of recombinant cytochrome P450 activity in metabolic pathways [letter]. Drug Metab Dispos 2003; 31: 1–2
Venkatakrishnan K, von Moltke LL, Court MH, et al. Comparison between cytochrome P450 (CYP) content and relative activity approaches to scaling from cDNA-expressed CYPs to human liver microsomes: ratios of accessory proteins as sources of discrepancies between the approaches. Drug Metab Dispos 2000; 28: 1493–504
Chang TK, Weber GF, Crespi CL, et al. Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res 1993; 53: 5629–37
Ren S, Yang JS, Kalhorn TF, et al. Oxidation of cyclophosphamide to 4-hydroxycyclophosphamide and deschloroethylcyclophosphamide in human liver microsomes. Cancer Res 1997; 57: 4229–35
Bohnenstengel F, Hofmann U, Eichelbaum M, et al. Characterization of the cytochrome P450 involved in side-chain oxidation of cyclophosphamide in humans. Eur J Clin Pharmacol 1996; 51: 297–301
Chen L, Waxman DJ, Chen D, et al. Sensitization of human breast cancer cells to cyclophosphamide and ifosfamide by transfer of a liver cytochrome P450 gene. Cancer Res 1996; 56: 1331–40
White IN, De Matteis F, Gibbs AH, et al. Species differences in the covalent binding of [14C]tamoxifen to liver microsomes and the forms of cytochrome P450 involved. Biochem Pharmacol 1995; 49: 1035–42
Innocenti F, Iyer L, Ratain MJ. Pharmacogenetics of anticancer agents: lessons from amonafide and irinotecan. Drug Metab Dispos 2001; 29: 596–600
Meisel C, Roots I, Cascorbi I, et al. How to manage individualized drug therapy: application of pharmacogenetic knowledge of drug metabolism and transport. Clin Chem Lab Med 2000; 38: 869–76
Brockmoller J, Kirchheiner J, Meisel C, et al. Pharmacogenetic diagnostics of cytochrome P450 polymorphisms in clinical drug development and in drug treatment. Pharmacogenomics 2000; 1: 125–51
Ma MK, Woo MH, McLeod HL. Genetic basis of drug metabolism. Am J Health Syst Pharm 2002; 59: 2061–9
Relling MV, Dervieux T. Pharmacogenetics and cancer therapy. Nat Rev Cancer 2001; 1: 99–108
Bachmann KA. Genotyping and phenotyping the cytochrome P-450 enzymes. Am J Ther 2002; 9: 309–16
Kivisto KT, Kroemer HK. Use of probe drugs as predictors of drug metabolism in humans. J Clin Pharmacol 1997; 37(1 Suppl.): 40–8S
Zhu B, Ou-Yang DS, Chen XP, et al. Assessment of cytochrome P450 activity by a five-drug cocktail approach. Clin Pharmacol Ther 2001; 70: 455–61
Conus P, Bondolfi G, Eap CB, et al. Pharmacokinetic fluvoxamine-clomipramine interaction with favorable therapeutic consequences in therapy-resistant depressive patient. Pharmacopsychiatry 1996; 29: 108–10
Baumann P, Broly F, Kosel M, et al. Ultrarapid metabolism of clomipramine in a therapy-resistant depressive patient, as confirmed by CYP2D6 genotyping [letter]. Pharmacopsychiatry 1998; 31: 72
Krynetski EY, Evans WE. Pharmacogenetics as a molecular basis for individualized drug therapy: the thiopurine S-methyltransferase paradigm. Pharm Res 1999; 16: 342–9
Huitema AD, Mathot RA, Tibben MM, et al. Validation of a therapeutic drug monitoring strategy for thiotepa in a high-dose chemotherapy regimen. Ther Drug Monit 2001; 23: 650–7
Huitema AD, Mathot RA, Tibben MM, et al. Validation of a therapeutic drug monitoring strategy for thiotepa in a high-dose chemotherapy regimen. Ther Drug Monit 2001; 23: 650–7
Rivory LP, Slaviero KA, Hoskins JM, et al. The erythromycin breath test for the prediction of drug clearance. Clin Pharmacokinet 2001; 40: 151–8
Sreerama L, Sladek NE. Primary breast tumor levels of suspected molecular determinants of cellular sensitivity to cyclophosphamide, ifosfamide, and certain other anticancer agents as predictors of paired metastatic tumor levels of these determinants: rational individualization of cancer chemotherapeutic regimens. Cancer Chemother Pharmacol 2001; 47: 255–62
Veenstra DL, Higashi MK, Phillips KA. Assessing the costeffectiveness of pharmacogenomics. AAPS PharmSci 2000; 2: E29
Jordan VC, Dix CJ, Rowsby L, et al. Studies on the mechanism of action of the nonsteroidal antioestrogen tamoxifen (I.C.I. 46,474) in the rat. Mol Cell Endocrinol 1977; 7: 177–92
Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 2003; 95(23): 1758–64
Johnson MD, Zuo H, Lee KH, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 2004; 85(2): 151–9
MacCallum J, Cummings J, Dixon JM, et al. Concentrations of tamoxifen and its major metabolites in hormone responsive and resistant breast tumours. Br J Cancer 2000; 82: 1629–35
Poon GK, Walter B, Lonning PE, et al. Identification of tamoxifen metabolites in human Hep G2 cell line, human liver homogenate, and patients on long-term therapy for breast cancer. Drug Metab Dispos 1995; 23: 377–82
Phillips DH. Understanding the genotoxicity of tamoxifen? Carcinogenesis 2001; 22: 839–49
Crewe HK, Ellis SW, Lennard MS, et al. Variable contribution of cytochromes P450 2D6, 2C9 and 3A4 to the 4-hydroxylation of tamoxifen by human liver microsomes. Biochem Pharmacol 1997; 53: 171–8
Dehal SS, Kupfer D. CYP2D6 catalyzes tamoxifen 4-hydroxylation in human liver. Cancer Res 1997; 57: 3402–6
Coller JK, Krebsfaenger N, Klein K, et al. The influence of CYP2B6, CYP2C9 and CYP2D6 genotypes on the formation of the potent antioestrogen Z-4-hydroxy-tamoxifen in human liver. Br J Clin Pharmacol 2002; 54: 157–67
Boocock DJ, Maggs JL, Brown K, et al. Major inter-species differences in the rates of O-sulphonation and O-glucuronylation of alpha-hydroxytamoxifen in vitro: a metabolic disparity protecting human liver from the formation of tamoxifen-DNA adducts. Carcinogenesis 2000; 21: 1851–8
Mani C, Kupfer D. Cytochrome P-450-mediated activation and irreversible binding of the antiestrogen tamoxifen to proteins in rat and human liver: possible involvement of flavin-containing monooxygenases in tamoxifen activation. Cancer Res 1991; 51: 6052–8
Mani C, Pearce R, Parkinson A, et al. Involvement of cytochrome P4503A in catalysis of tamoxifen activation and covalent binding to rat and human liver microsomes. Carcinogenesis 1994; 15: 2715–20
Divi RL, Dragan YP, Pitot HC, et al. Immunohistochemical localization and semi-quantitation of hepatic tamoxifen-DNA adducts in rats exposed orally to tamoxifen. Carcinogenesis 2001; 22: 1693–9
Boocock DJ, Brown K, Gibbs AH, et al. Identification of human CYP forms involved in the activation of tamoxifen and irreversible binding to DNA. Carcinogenesis 2002; 23: 1897–901
Moorthy B, Sriram P, Randerath E, et al. Effects of cytochrome P450 inducers on tamoxifen genotoxicity in female mice in vivo. Biochem Pharmacol 1997; 53: 663–9
Comoglio A, Gibbs AH, White IN, et al. Effect of tamoxifen feeding on metabolic activation of tamoxifen by the liver of the rhesus monkey: does liver accumulation of inhibitory metabolites protect from tamoxifen-dependent genotoxicity and cancer? Carcinogenesis 1996; 8: 1687–93
Carter SJ, Li XF, Mackey JR, et al. Biomonitoring of urinary tamoxifen and its metabolites from breast cancer patients using nonaqueous capillary electrophoresis with electrospray mass spectrometry. Electrophoresis 2001; 22: 2730–6
Clarke R, Skaar TC, Bouker KB, et al. Molecular and pharmacological aspects of antiestrogen resistance. J Steroid Biochem Mol Biol 2001; 76: 71–84
Osborne CK, Jarman M, McCague R, et al. The importance of tamoxifen metabolism in tamoxifen-stimulated breast tumor growth. Cancer Chemother Pharmacol 1994; 34: 89–95
Crewe HK, Notley LM, Wunsch RM, et al. Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4t′-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos 2002; 30: 869–74
Williams ML, Lennard MS, Martin IJ, et al. Interindividual variation in the isomerization of 4-hydroxytamoxifen by human liver microsomes: involvement of cytochromes P450. Carcinogenesis 1994; 15: 2733–8
Sharma M, Shubert DE, Sharma M, et al. Biotransformation of tamoxifen in a human endometrial expiant culture model. Chem Biol Interact 2003; 146(3): 237–49
McFadyen MC, Breeman S, Payne S, et al. Immunohistochemical localization of cytochrome P450 CYP1B1 in breast cancer with monoclonal antibodies specific for CYP1B1. J Histochem Cytochem 1999; 47: 1457–64
Parekh H, Simpkins H. The transport and binding of taxol. Gen Pharmacol 1997; 29: 167–72
O’Leary J, Volm M, Wasserheit C, et al. Taxanes in adjuvant and neoadjuvant therapies for breast cancer. Oncology 1998; 12: 23–7
Kumar G, Ray S, Walle T, et al. Comparative in vitro cytotoxic effects of taxol and its major human metabolite 6 alphahydroxytaxol. Cancer Chemother Pharmacol 1995; 36: 129–35
Harris JW, Katki A, Anderson LW, et al. Isolation, structural determination, and biological activity of 6 alpha-hydroxytaxol, the principal human metabolite of taxol. J Med Chem 1994; 37: 706–9
Sonnichsen DS, Liu Q, Schuetz EG, et al. Variability in human cytochrome P450 paclitaxel metabolism. J Pharmacol Exp Ther 1995; 275: 566–75
Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos 1998; 26: 229–33
Cresteil T, Monsarrat B, Alvinerie P, et al. Taxol metabolism by human liver microsomes: identification of cytochrome P450 isozymes involved in its biotransformation. Cancer Res 1994; 54: 386–92
Rahman A, Korzekwa KR, Grogan J, et al. Selective biotransformation of taxol to 6 alpha-hydroxytaxol by human cytochrome P450 2C8. Cancer Res 1994; 54: 5543–6
Mechetner E, Kyshtoobayeva A, Zonis S, et al. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin Cancer Res 1998; 4: 389–98
Sarris AH, Younes A, McLaughlin P, et al. Cyclosporin A does not reverse clinical resistance to paclitaxel in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol 1996; 14: 233–9
Miller TP, Chase EM, Dorr R, et al. A phase I/II trial of paclitaxel for non-Hodgkin’s lymphoma followed by paclitaxel plus quinine in drug-resistant disease. Anticancer Drugs 1998; 9: 135–40
Brinkmann U. Functional polymorphisms of the human multidrug resistance (MDR1) gene: correlation with P glycoprotein expression and activity in vivo. Novartis Found Symp 2002; 243: 207–10
Yu D, Liu B, Jing T, et al. Overexpression of both p185c-erbB2 and p170mdr-l renders breast cancer cells highly resistant to taxol. Oncogene 1998; 16: 2087–94
Huang Y, Ibrado AM, Reed JC, et al. Co-expression of several molecular mechanisms of multidrug resistance and their significance for paclitaxel cytotoxicity in human AML HL-60 cells. Leukemia 1997; 11: 253–7
Kavallaris M, Kuo DYS, Burkhart CA, et al. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest 1997; 100: 1282–93
Giannakakou P, Sackett DL, Kang YK, et al. Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem 1997; 272: 17118–25
Monzo M, Rosell R, Sanchez JJ, et al. Paclitaxel resistance in non-small-cell lung cancer associated with beta-tubulin gene mutations. J Clin Oncol 1999; 17: 1786–93
Ohta S, Nishio K, Kubota N, et al. Characterization of a taxolresistant human small-cell lung cancer cell line. Jpn J Cancer Res 1994; 85: 290–7
Parekh H, Simpkins H. Species-specific differences in taxol transport and cytotoxicity against human and rodent tumor cells: evidence for an alternate transport system. Biochem Pharmacol 1996; 51: 301–11
Parekh H, Wiesen K, Simpkins H. Acquisition of taxol resistance via P-glycoprotein- and non-P-glycoprotein-mediated mechanisms in human ovarian carcinoma cells. Biochem Pharmacol 1997; 53: 461–70
Kern DH. Heterogeneity of drug resistance in human breast and ovarian cancers. Cancer J Sci Am 1998; 4: 41–5
Lee DK, Kim YH, Kim JS, et al. Induction and characterization of taxol-resistance phenotypes with a transiently expressed artificial transcriptional activator library. Nucleic Acids Res 2004; 32(14): E1–16
Savage DG, Antman KH. Imatinib mesylate: a new oral targeted therapy. N Engl J Med 2002; 346: 683–93
O’Dwyer ME, Druker BJ. STI571: an inhibitor of the BCR-ABL tyrosine kinase for the treatment of chronic myelogenous leukaemia. Lancet Oncol 2000; 1: 207–11
Buchdunger E, Matter A, Druker BJ. Bcr-Abl inhibition as a modality of CML therapeutics. Biochim Biophys Acta 2001; 1551: M11–8
Verweij J, Judson I, van Oosterom A. STI571: a magic bullet? Eur J Cancer 2001; 37: 1816–9
Fabbro D, Ruetz S, Buchdunger E, et al. Protein kinases as targets for anticancer agents: from inhibitors to useful drugs. Pharmacol Ther 2002; 93: 79–98
Apperley JF, Gardembas M, Melo JV, et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Engl J Med 2002; 347: 481–7
Cohen MH, Williams G, Johnson JR, et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res 2002; 8: 935–42
Kantarjian HM, Cortes J, O’Brien S, et al. Imatinib mesylate (STI571) therapy for Philadelphia chromosome-positive chronic myelogenous leukemia in blast crisis. Blood 2002; 99: 3547–53
Krystal GW. Mechanisms of resistance to imatinib (STI571) and prospects for combination with conventional chemotherapeutic agents. Drug Resist Updat 2001; 4: 16–21
McCormick F. New-age drug meets resistance. Nature 2001; 412: 281–2
Shah N, Nicoll J, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2002; 2: 117–25
Roumiantsev S, Shah NP, Gorre ME, et al. Clinical resistance to the kinase inhibitor STI-571 in chronic myeloid leukemia by mutation of Tyr-253 in the Abl kinase domain P-loop. Proc Natl Acad Sci U S A 2002; 99: 10700–5
Hofmann WK, Jones LC, Lemp NA, et al. Ph (+) acute lymphoblastic leukemia resistant to the tyrosine kinase inhibitor STI571 has a unique BCR-ABL gene mutation. Blood 2002; 99: 1860–2
Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001; 293: 876–80
Von Bubnoff N, Schneller F, Peschel C, et al. BCR-ABL gene mutations in relation to clinical resistance of Philadelphiachromosome-positive leukaemia to STI571: a prospective study. Lancet 2002; 359: 487–91
Branford S, Rudzki Z, Walsh S, et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood 2002; 99: 3472–5
Le Coutre P, Tassi E, Varella-Garcia M, et al. Induction of resistance to the Abelson inhibitor STI571 in human leukemic cells through gene amplification. Blood 2000; 95: 1758–66
Keeshan K, Mills KI, Cotter TG, et al. Elevated Bcr-Abl expression levels are sufficient for a haematopoietic cell line to acquire a drug-resistant phenotype. Leukemia 2001; 15: 1823–33
Mahon FX, Deininger MW, Schultheis B, et al. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood 2000; 96: 1070–9
Hegedus T, Orfi L, Seprodi A, et al. Interaction of tyrosine kinase inhibitors with the human multidrug transporter proteins, MDR1 and MRP1. Biochim Biophys Acta 2002; 1587: 318–25
Illmer T, Schaich M, Platzbecker U, et al. P-glycoproteinmediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylate. Leukemia 2004; 18: 401–8
Ozvegy-Laczka C, Hegedus T, Varady G, et al. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol 2004; 65(6): 1485–95
Le Coutre P, Kreuzer KA, Il-Kang NA. Determination of alpha-1 acid glycoprotein in patients with Ph+ chronic myeloid leukemia during the first 13 weeks of therapy with STI571. Blood Cells Mol Dis 2002; 28: 75–85
Gambacorti-Passerini C, Barni R, Le Coutre P, et al. Role of alphal acid glycoprotein in the in vivo resistance of human BCR-ABL (+) leukemic cells to the abl inhibitor STI571. J Natl Cancer Inst 2000; 92: 1641–50
Larghero J, Leguay T, Mourah S, et al. Relationship between elevated levels of the alpha 1 acid glycoprotein in chronic myelogenous leukemia in blast crisis and pharmacological resistance to imatinib (Gleevec) in vitro and in vivo. Biochem Pharmacol 2003; 66(10): 1907–13
Gambacorti-Passerini CB, Rossi F, Verga M, et al. Differences between in vivo and in vitro sensitivity to imatinib of Bcr/Abl+ cells obtained from leukemic patients. Blood Cells Mol Dis 2002; 28: 361–72
Gambacorti-Passerini C, Zucchetti M, Russo D, et al. Alphal acid glycoprotein binds to imatinib (STI571) and substantially alters its pharmacokinetics in chronic myeloid leukemia patients. Clin Cancer Res 2003; 9(2): 625–32
Tipping AJ, Deininger MW, Goldman JM, et al. Comparative gene expression profile of chronic myeloid leukemia cells innately resistant to imatinib mesylate. Exp Hematol 2003; 31: 1073–80
McLean LA, Gatmann I, Capdeville R, et al. Pharmacogenomic analysis of cytogenetic response in chronic myeloid leukemia patients treated with imatinib. Clin Cancer Res 2004; 10: 155–65
Kikuta Y, Yamashita Y, Kashiwagi S, et al. Expression and induction of CYP4F subfamily in human leukocytes and HL60 cells. Biochim Biophys Acta 2004; 1683(1–3): 7–15
CDER new and generic drug approvals: 1998–2004 [online]. Available from URL: http://www.fda.gov/cder/approval/index.htm [Accessed 2005 Mar 10]
Lin YS, Lockwood GF, Graham MA, et al. In-vivo phenotyping for CYP3A by a single-point determination of midazolam plasma concentration. Pharmacogenetics 2001; 11: 781–91
Baron JM, Zwadlo-Klarwasser G, Jugert F, et al. Cytochrome P450 1B1: a major P450 isoenzyme in human blood monocytes and macrophage subsets. Biochem Pharmacol 1998; 56: 1105–10
Spencer DL, Masten SA, Lanier KM, et al. Quantitative analysis of constitutive and 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced cytochrome P450 1B1 expression in human lymphocytes. Cancer Epidemiol Biomarkers Prev 1999; 8: 139–46
Finnstrom N, Ask B, Dahl ML, et al. Intra-individual variation and sex differences in gene expression of cytochromes P450 in circulating leukocytes. Pharmacogenomics J 2002; 2: 111–6
Bernauer U, Garritsen H, Heinrich-Hirsch B, et al. Immunochemical analysis of cytochrome P450 variability in human leukapheresed samples and its consequences for the risk assessment process. Regul Toxicol Pharmacol 2003; 37(2): 318–27
Al-Ali HK, Heinrich MC, Lange T, et al. High incidence of BCR-ABL kinase domain mutations and absence of mutations of the PDGFR and KIT activation loops in CML patients with secondary resistance to imatinib. Hematol J 2004; 5(1): 55–60
Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood 2003; 102(1): 276–83
Barthe C, Cony-Makhoul P, Melo JV, et al. Roots of clinical resistance to STI-571 cancer therapy. Science 2001; 293: 2163
Hochhaus A, Kreil S, Corbin A, et al. Roots of clinical resistance to STI-571 cancer therapy. Science 2001; 293: 2163
Roche-Lestienne C, Soenen-Cornu V, Grardel-Duflos N, et al. Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood 2002; 100: 1014–8
Shah NP, Sawyers CL. Mechanisms of resistance to STI571 in Philadelphia chromosome-associated leukemias. Oncogene 2003; 22(47): 7389–95
McLeod HL. Individualized cancer therapy: molecular approaches to the prediction of tumor response. Expert Rev Anticancer Ther 2002; 2: 113–9
Peters GJ, Backus HH, Freemantle S, et al. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta 2002 Jul 18; 1587: 194–205
McLeod HL, Siva C. The thiopurine S-methyltransferase gene locus: implications for clinical pharmacogenomics. Pharmacogenomics 2002; 3: 89–98
Salonga D, Danenberg KD, Johnson M, et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine Phosphorylase. Clin Cancer Res 2000; 6: 1322–7
Scappini B, Gatto S, Onida F, et al. Changes associated with the development of resistance to imatinib (STI571) in two leukemia cell lines expressing p210 Bcr/Abl protein. Cancer 2004; 100(7): 1459–71
Nimmanapalli R, O’Bryan E, Huang M, et al. Molecular characterization and sensitivity of STI-571 (imatinib mesylate, Gleevec)-resistant, Bcr-Abl-positive, human acute leukemia cells to SRC kinase inhibitor PD180970 and 17-allylamino-17-demethoxygeldanamycin. Cancer Res 2002; 62(20): 5761–9
Donate NJ, Wu JY, Stapley J, et al. Imatinib mesylate resistance through BCR-ABL independence in chronic myelogenous leukemia. Cancer Res 2004; 64(2): 672–67
Hochhaus A, Kreil S, Corbin AS, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia 2002; 16: 2190–6
Hofmann WK, de Vos S, Elashoff D, et al. Relation between resistance of Philadelphia-chromosome-positive acute lymphoblastic leukaemia to the tyrosine kinase inhibitor STI571 and gene-expression profiles: a gene-expression study. Lancet 2002; 359: 481–6
Hoover RR, Mahon FX, Melo JV, et al. Overcoming STI571 resistance with the farnesyl transferase inhibitor SCH66336. Blood 2002; 100: 1068–71
Luzzatto L, Melo JV. Acquired resistance to imatinib mesylate: selection for pre-existing mutant cells [letter]. Blood 2002; 100: 1105
Acknowledgements
The author is grateful to Howard L. McLeod and William D. Figg for advising to write this review article, to Laurent Décosterd and Thierry Buclin for their fruitful discussions and to Catherine Maass for the assistance in writing this review. No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rochat, B. Role of Cytochrome P450 Activity in the Fate of Anticancer Agents and in Drug Resistance. Clin Pharmacokinet 44, 349–366 (2005). https://doi.org/10.2165/00003088-200544040-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200544040-00002