Abstract
Synopsis
Defibrotide is a deoxyribonucleic acid derivative extracted from mammalian organs, which has been developed for the treatment of a number of vascular disorders. It appears to increase fibrinolysis and may possess antithrombotic, antiatherosclerotic and anti-ischaemic actions, probably due to its ability to selectively increase prostaglandin I2 and E2 levels and to increase tissue plasminogen activator and decrease plasminogen activator inhibitor function. Defibrotide is available as an intravenous and intramuscular preparation, and also as an oral formulation for long term use.
Trials performed to date have provided initial evidence that defibrotide is effective in the treatment of peripheral obliterative arterial disease and acute thrombophlebitis, while preliminary data suggest possible use in preventing fibrin deposition in the circuitry of renal haemodialysis equipment. Efficacy in preventing deep vein thrombosis after surgery has been demonstrated but defibrotide does not appear to offer any therapeutic advantage over heparin. Further clinical experience is required in other disorders, including acute myocardial infarction, Raynaud’s phenomenon, renal thrombotic microangiopathy and renal transplant rejection, before adequate assessment of efficacy in these areas can be made.
Defibrotide is well tolerated, as assessed in trials of up to 6 months duration, with a low global incidence of adverse events (<1 to 9%). Mild allergic reactions and gastrointestinal disturbances have occasionally been described, and a hypotensive effect has also infrequently been observed.
Thus, available data suggest that deflbrotide is a well tolerated agent with little or no anticoagulant activity, which is conveniently available in both parenteral and oral formulations. Initial data indicate that the drug may be a useful alternative in the treatment of peripheral obliterative arterial disease and thrombophlebitis, while its therapeutic potential in other vascular disorders and efficacy relative to established agents remains to be fully determined.
Pharmacodynamic Properties
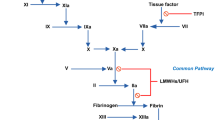
Defibrotide appears to have a number of effects on mediators of the coagulation and fibrinolytic systems. The drug selectively stimulates prostaglandin I2 and E2 production and, therefore, increases blood levels of these prostanoids. This appears to be a selective action, as thromboxane A2 levels are unaffected. Defibrotide also increases the function of tissue plasminogen activator while decreasing that of plasminogen activator inhibitor, thus increasing fibrinolytic activity. Recent data indicate that defibrotide has some affinity for adenosine receptors, an action which may be responsible for the drug’s ability to increase prostaglandin I2 levels and prevent leucocyte activation and subsequent release of oxygen free radicals. Furthermore, a specific binding site for defibrotide on the vascular endothelium has recently been identified, although the clinical relevance of this has yet to be clarified.
Defibrotide appears to be largely devoid of anticoagulant properties as determined by a lack of clinically significant effects on coagulation parameters, including partial and activated thromboplastin times and prothrombin time. Data concerning the influence of defibrotide on specific clotting factors are incomplete, but the drug appears to have no effect on von Willebrand factor, factor VIII, factor Xa and prekallikrein, while its effects on antithrombin-III, fibrinogen and Protein C require further clarification. Defibrotide has been reported to have little effect on platelet numbers, but may inhibit platelet function, possibly by stimulating the formation of prostaglandin I2 or increasing platelet cyclic adenosine monophosphate levels.
Many of the pharmacodynamic effects of defibrotide are likely to be of clinical relevance. An increase in prostaglandin E2 and I2 levels may have therapeutic actions by causing regional vasodilation, inhibition of platelet aggregation and reduction of leukotriene B4 levels (a possible mediator of ischaemic damage). Inhibition of leucocyte activation may reduce the production of free radicals which may slow the progression of atheroma formation and prevent post-ischaemic damage.
Thus, animal studies have provided evidence that defibrotide has in vivo anti-ischaemic, antiatherosclerotic and antithrombotic effects. These studies have prompted clinical trials in a large number of vascular disorders and indicated potential uses of defibrotide in the future.
Pharmacokinetic Properties
Investigation of the pharmacokinetic behaviour of defibrotide is difficult because the drug is degraded in the body to a number of products and the identity of the active derivative in humans is unclear. To date, the majority of pharmacokinetic data have been determined by following the fate of the carbohydrate moiety of the drug, 6-deoxyribose, as a marker of the parent compound, although a recent study has used radiolabelled defibrotide. Due to these difficulties the data regarding the pharmacokinetics of defibrotide in humans (obtained from 23 healthy volunteers and 14 patients) are incomplete.
Intravenous injection of defibrotide 0.5 to 16 mg/kg in healthy volunteers results in a dose-dependent increase in plasma 6-deoxyribose concentrations. Steady-state plasma 6-deoxyribose concentrations of 25 to 68 mg/L were achieved after intravenous infusion of defibrotide (100 to 400 mg/h after a bolus injection of 200 to 400mg) or a single intravenous injection of 200mg. Plasma concentrations of 6-deoxyribose are maintained during the infusion period and subsequently decrease rapidly to basal concentrations at the end of infusion. The drug is absorbed after oral administration, giving maximum plasma concentrations of up to 7.8 mg/L 0.5 hours after a dose of [125I]defibrotide 423mg in patients with cancer. There does not appear to be any accumulation of defibrotide after repeated administration as areas under the plasma concentration-time curves were similar after the first and last doses administered to rats.
Volume of distribution values of 0.04 to 0.05 L/kg were obtained after intravenous administration of defibrotide 0.5 to 16 mg/kg to healthy volunteers. Data from animal studies indicate that after oral and intravenous administration the highest concentrations of defibrotide are found in the blood components, aorta, bone marrow, spleen, thyroid, adrenal glands and skin and highly perfused organs such as the liver and kidneys. The low bioavailability of defibrotide in humans (2.6 to 13%) may be due to uptake of the drug by the vascular endothelium.
Elimination of defibrotide in humans follows different kinetic models depending on the dose, with a 1-compartment model being the most appropriate following administration of low doses and a 2-compartment model better suited following high doses. The elimination half-life is short and increases with dose, with values of between 9.8 and 27.1 minutes after intravenous doses of 0.5 to 16 mg/kg or single intravenous injection of 200mg. The elimination half-life appears to be independent of route of administration with similar values being obtained after oral and intravenous administration. Animal and human studies have demonstrated that defibrotide is eliminated via the urine and the faeces, with a greater proportion of a radioactive dose undergoing renal elimination compared with biliary elimination following intravenous administration compared with oral administration. The mechanism of elimination is thought to consist of a non-saturable renal process and a saturable hepatic process.
Therapeutic Use
In patients with peripheral obliterative arterial disease defibrotide is effective in increasing absolute walking distance, a reliable measure of arterial sufficiency, with significant increases in the range 41 to 61% compared with baseline. Furthermore, defibrotide appears to be as effective as buflomedil and indobufen in improving this parameter. An improvement of regional limb blood flow has also been indicated. Despite the apparent efficacy of the drug in reducing symptoms of peripheral obliterative arterial disease, long term administration is required to determine whether defibrotide can influence the evolution and progression of the disease.
Significant decreases (43 to 99%) in the severity of symptoms of phlebitis, such as redness, pain, swelling and heat, have been reported following administration of defibrotide, indicating that the drug is an effective treatment in this indication. Comparative trials with heparin suggest that defibrotide shows similar efficacy to this established treatment. However, these encouraging data require further substantiation in trials using less subjective criteria of efficacy.
The use of defibrotide as an alternative to heparin in renal haemodialysis, especially in patients in whom heparin is contraindicated, has also been investigated. Preliminary results suggest that defibrotide is effective in preventing fibrin deposition in the circuitry of dialysis machines.
Despite encouraging initial data, the efficacy of defibrotide as a treatment for acute myocardial infarction, either as a monotherapy or in conjunction with standard thrombolytic agents, cannot be assessed as yet because of small patient numbers and a lack of comparative data. A similar lack of substantial data hampers assessment of the effect of defibrotide in renal disorders such as thrombotic microangiopathy and renal transplant rejection, and in Raynaud’s phenomenon. Defibrotide has been assessed as a prophylactic therapy against deep vein thrombosis and, although more effective than placebo, the drug does not appear to offer any therapeutic advantage over low dose heparin.
Tolerability
Defibrotide appears to be well tolerated with a global incidence of adverse events reported to range from < 1 to 9%. Adverse events were mostly mild and rarely resulted in patients withdrawing from treatment. An allergic reaction, characterised by sweating, tachycardia, rash, erythema and pruritus, has been described. The dermatological manifestation can be general or localised to the site of injection. A low incidence of gastrointestinal disturbance, such as nausea, vomiting and abdominal pain, and isolated occurrences of fever, headache, dizziness, palpitations, back pain and oedema have also been observed. A hypotensive effect has been reported in a limited number of patients.
Defibrotide has little or no effect on haemostatic components and, therefore, does not appear to have a major anticoagulant action. It has been shown to be associated with a lower incidence of operative and postoperative haemorrhagic complications than heparin.
Few data are available concerning the possible interaction of defibrotide with other drugs. However, caution is advised during the use of a combination of defibrotide and heparin as this treatment appears to result in a greater anticoagulant effect than the use of heparin alone.
Dosage and Administration
Defibrotide is available in intravenous, intramuscular and oral formulations. Parenteral routes of administration have been used during the treatment of disorders which require short term therapy and in haemodialysis patients, whereas the oral route has been used during long term treatment in order to improve patient acceptability.
The dosage of defibrotide administered for most disorders has commonly been between 400 and 1200 mg/day, with 800 mg/day the most frequently used. Higher dosages of up to 5.6 g/day have been administered for the treatment of acute myocardial infarction. Intravenous dosing regimens have consisted of an initial bolus injection followed by a slow infusion, and both parenteral and oral formulations have been divided into 2 to 4 doses. Defibrotide has been used as prophylaxis against the formation of deep vein thrombosis during and following surgery, with therapy commonly initiated the day before surgery.
Similar content being viewed by others
References
Alberico P, Tettamanti R, Bianchi G, Porta R, Mantovani M, et al. Stimulation of adenosine receptors and anti-ischaemic efficacy of defibrotide (2): blunting of leukcocyte adherence in rats. Abstract. International Journal of Purine and Pyrimidine Research 3: 164, 1992
Annoni F, Ceva M, Gerosa C. Preventing deep venous thrombosis after major abdominal surgery. In Italian. Minerva Chirurgica 45: 881–882, 1990
Anonymous. Prevention of deep venous thrombosis and pulmonary embolism. Lancet I: 1202–1203, 1986
Aoki N, Seigfried M, Tsao P, Lento P, Lefer AM. Beneficial mechanism of action of a prostacyclin enhancing agent in splanchnic artery occlusion shock. Research Communications in Chemical Pathology and Pharmacology 60: 275–289, 1988
Arosio E, Pancera P, Zannoni M, Arcaro G, Priante F, et al. Defibrotide and peripheral obliterative arterial disease: preliminary data. International Journal of Clinical Pharmacology, Therapy and Toxicology 27: 526–529, 1989
Avellone G, Mandalà V, Pinto A, Martino A, Strano A. Clinical evaluation of short-term defibrotide treatment of patients with atherosclerosis obliterans of the lower limbs. Haemostasis 16 (Suppl. 1): 55–58, 1986
Balkuv-Ulutin S, Ulutin T, Kaya G, Yardimci T, Ulutin ON. The effect of defibrotide on protein C levels in human (preliminary results). In Ulutin & Vinazzer (Eds) Thrombosis and haemorrhagic diseases, pp. 409–410, Goezlem Printing, Istanbul, 1986
Barone D, Salvetti L, Bianchi G, Calvani AB, Mantovani M, et al. On the molecular site of action of defibrotide. Abstract. Pharmacological Research 25: 123–124, 1992
Baumann I, Baumann J, Strobach H. Responses to defibrotide in human platelets loaded with the fluorescent calcium indicator indo-1am. Naunyn Schmiedeberg’s Archives of Pharmacology 343 (Suppl.): 44, 1991
Belcaro G. Vasospasm in intermittent claudication. Current Therapeutic Research 48: 667–675, 1990a
Belcaro G. Evolution of superficial vein thrombosis treated with defibrotide: comparison with low dose subcutaneous heparin. International Journal of Tissue Reactions 1. 2: 319–324, 1990b
Berne RM, The role of adenosine in the regulation of coronary blood flow. Circulation Research 47: 807–813, 1980
Berti F, Rossoni G, Biasi G, Buschi A, Mandelli V, et al. Defibrotide, by enhancing prostacyclin generation, prevents endothelin-1 induced contraction in human saphenous veins. Prostaglandins 40: 337–350, 1990
Berti F, Rossoni G, Bianchi G, Alberico P, Tettamanti R et al. Effect of defibrotide on prostacyclin release from isolated rabbit kidneys and protection from post-ischemic acute renal failure in vivo. Eicosanoids 4: 209–215, 1991
Berti F, Rossoni G, Niada R, Folco G, Omini C et al. Defibrotide, an antithrombotic substance that preserves postsynaptic α- and β-adrenergic function in post acute infarcted rabbit hearts. Journal of Cardiovascular Pharmacology 8: 235–240, 1986
Bertoldo U, Alberghina A, Verna C, Pessione E, Olivero G. Activity of defibrotide in the prevention of deep vein thrombosis. In Italian. Minerva Chirurgica 43: 57–60, 1988
Biagi G, Legnani C, Rodorigo G, Coccheri S. Modulation of arachidonate metabolite generation in human blood by oral defibrotide. Arzneimittel-Forschung/Drug Research 41: 511–514, 1991
Bianchi G, Mantovani M, Prino G, Salvetti L, Barone P. Possible agonistic interaction of defibrotide, a DNA derivative, with adenosine receptors ‘in vitro’. International Symposium on Pharmacology of Purinergic Receptors. Implications for Drug Design, Noordwijk, The Netherlands, July 6–8, 1990. Abstract P7, 1990
Bianchi G, Calvani AB, Mantovani M, Prino G. Enhancement by defibrotide of prostanoid neosynthesis from arachidonic acid: an adenosine receptor-mediated effect? Seminars in Thrombosis and Hemostasis 17: 399–403, 1991
Bilsel S, Taga Y, Yalgin AS, Emerk K, Ulutin ON. Effect of defibrotide and endothelial cell growth supplement on human endothelial cells in culture. Hematology Reviews 3: 29–34, 1989
Bilsel S, Süha Yaiçin A, Taga Y, Emerk K. Interaction of 3H-defibrotide with cultured human umbilical vein endothelial cells. Thrombosis Research 58: 455–460, 1990
Bilsel S, Ersahin Ç, Taga Y, Emerk K. Subcellular localization of radioactively labelled defibrotide in cultured endothelial cells. Thrombosis Research 66: 385–390, 1992
Bitterman H, Lefer DJ, Lefer AM. Novel mechanism of action of a prostacyclin enhancing agent in haemorrhagic shock. Naunyn-Schmiedeberg’s Archives of Pharmacology 337: 679–686, 1988
Bonomini V, Frascà GM, Raimondi C, Liviano D’arcangelo G, Vangelista A. Effect of a new antithrombotic agent (defibrotide) in acute renal failure due to thrombotic microangiopathy. Nephron 40: 195–200, 1985
Bowman WC, Rand MJ. Textbook of Pharmacology, 2nd edition, Blackwell Scientific Publications, 1988
Bracale G, Iandoli R. Effects of defibrotide in patients with chronic peripheral occlusive arteriopathy of the lower limbs: a controlled study versus buflomedil. Current Therapeutic Research 51: 882–888, 1992
Breddin HK, Krupinski K, Fareed J. On the effect of defibrotide on platelet function, coagulation on thrombus formation in rat mesenteric arterioles or venules. Abstract. Thrombosis and Haemostasis 62: 429, 1989
Buccianti G, Valenti G, Lorenz M, Cresseri D, Strada E, et al. Kinetics of anti-Xa activity during combined defibrotide — heparin administration in hemodialysis. International Journal of Artificial Organs 13: 416–420, 1990
Burton K. A study of the conditions and mechanisms of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochemistry 62: 315–323, 1956
Carelli M, Citone C, Di Marcotullio G, Giampaolo P, Malinconico U, et al. A pilot evaluation of the effects of defibrotide in patients affected by acute myocardial infarction. Seminars in the Thrombosis and Hemostasis 14 (Suppl.): 71–76, 1988
Caruana RJ, Raja RM, Bush JV, Kramer MS, Goldstein SJ. Heparin free dialysis: comparative data and results in high risk patients. Kidney International 31: 1351–1355, 1987
Chiurazzi B, Mozzi E, Spinola A, Germiniani R, Montorsi P. Use of defibrotide in prophylaxis of deep vein thrombosis in general surgery. International Angiology 4 (Suppl.): 55–56, 1985
Ciavarella N, Ettorre C, Schiavoni M, Schonauer S, Cicinelli E, et al. Effectiveness of defibrotide for prophylaxis of deep venous thrombosis in gynecological surgery: a double-blind, placebo-controlled clinical trial. Haemostasis 16 (Suppl. 1): 39–41,1986
Cimminiello C, Milani M, Pietra A, Rossi F, Aloisio M, et al. Deficient fibrinolytic response in patients with Raynaud’s phenomenon and its correction with defibrotide. Seminars in Thrombosis and Hemostasis 17 (Suppl.): 106–111, 1991
Cirillo F, Margaglione M, Vecchione G, Ames PRJ, Coppola A, et al. In vitro inhibition by defibrotide of monocyte Superoxide anion generation. A possible mechanism for the antithrombotic effect of a polydeoxyribonucleotide-derived drug. Haemostasis 21: 98–105, 1991
Çizmeci G. In vivo effects of defibrotide on platelet c-AMP and blood prostanoid levels. Haemostasis 16 (Suppl.): 31–35, 1986
Çizmeci G, Ulutin ON. Prostanoid synthesis in isolated leucocytes and in-vitro effect of defibrotide. In Ulutin & Vinazzer (Eds) Thrombosis and hemorrhagic diseases. Proceedings of the IVth international meeting of Danubian League against Thrombosis and Hemorrhagic Diseases, pp. (199–204), Gözlem Matbaacihk Koll, Istanbul, 1986
Coccheri S, Biagi G, Legnani C, Bianchini B, Grauso F. Acute effects of defibrotide, an experimental antithrombotic agent, on fibrinolysis and blood prostanoids in man. European Journal of Clinical Pharmacology 35: 151–156, 1988
Coccheri S, De Rosa V, Dettori AG, Ponari O, Bizzi B, et al. Effect on fibrinolysis of a new antithrombotic agent: fraction P (Defibrotide). A multicentre trial. International Journal of Clinical Pharmacology Research 3: 227–245, 1982
Coccheri S, De Rosa V, Pelusi G, Moretti B, Camerini T, et al. The effect of some fibrinolitically active sulfated polysaccharides and a polydesoxynucleotide on the inhibition of factor Xa. Urinalysis and Thrombolysis 10: 61–67, 1985
Coffman JD. Intermittent claudication: not so benign. American Heart Journal 1127-1128, 1986
Colditz GA, Tuden RL, Oster G. Rates of venous thrombosis after general surgery: combined results of randomised clinical trials. Lancet II: 143–145, 1986
Cornelli U, Nazzari M. Defibrotide: An overview of clinical pharmacology and early clinical studies. Seminars in Thrombosis and Hemostasis 14 (Suppl.): 64–70, 1988
Corsi C, Pollastri M, Cipriani C, Leanza D, Nazzari M, Gerosa C. Defibrotide in the treatment of superficial thrombophlebitis. In Italian. Giornale Italiano di Cardiologia 9: 95–98, 1989
Costantini V, Luzi P, Boselli C, Bastiano ML, Bisacci R, et al. Effects of defibrotide on prostanoid synthesis and fibrinolytic activity in human veins. European Journal of Internal Medicine 1: 115–120, 1989
Craveri A, Tornaghi G, Ranieri R, Stanzani M, Landi G, et al. Effects of defibrotide after oral and parenteral administration in patients with peripheral obliterative arterial disease (POAD). International Angiology 9: 274–277, 1990
Cremonese M, Rigon N, Ratti G, Pojana A, Todesco A, et al. Prevention of thromboembolism in patients submitted to hip arthroplasty. In Italian. Minerva Medica 79: 367–372, 1988
Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, et al. The prevalence of peripheral arterial disease in a defined population. Circulation 71: 510–515, 1985
Davi G, Catalano I, Belvedere M, Amato S, Mogavero A, et al. Effects of defibrotide on fibrinolytic activity in diabetic patients with stable angina pectoris. Thrombosis and Research 65: 211–220, 1992
De Luca N, Rosiello G, Lembo G, Crispino MR, Vecchioni F, et al. Treating peripheral artery disease of atherosclerotic nature with orally administered defibrotide: a double-blind controlled study versus placebo. In Italian. Medical Praxis 8: 1–8, 1987
Del Maschio A, Evangelista V, Rajtar G, Chen Z, et al. Platelet activation by polymorphonuclear leukocytes exposed to chemotactic agents. American Journal of Physiology 258: H870–879, 1990
Dina F, Calvani AB, Ferrari PA, Gerosa C, Nazzari M, et al. Treatment of acute thrombophlebitis with defibrotide — a multicentre study. In Italian. II Gironale Italiano di Medicini 111: 191–197, 1988 aiDi Perri T, Laghi Pasini F. Effect of defibrotide on polymorphonuclear leukocytes: modulation of calcium entry and availability. Seminars in Thrombosis and Hemostasis 14 (Suppl.): 23–26, 1988
Di Perri T, Vittoria A, Messa GL, Cappelli R. Defibrotide therapy for thrombophlebitis — controlled clinical trial. Haemostasis 16 (Suppl. 1): 42–47, 1986
Di Perri T, Laghi Pasini F, Ceccatelli L, Pasqui AL. Defibrotide inhibits Ca2+ dependent neutrophil activation: implications for its pharmacological activity in vascular disorders. Angiology — The Journal of Vascular Diseases: 971–976, 1991
Di Perri T, Laghi Pasini F, Frigerio C, Capecchi PL, Messa GL, et al. New in vivo model to assess venous endothelial cell functions. Effect of defibrotide. European Journal of Clinical Pharmacology 37: 351–357, 1989
Ernst E, Hammerschmidt DE, Bagge U, Matrai A, Dormandy JA. Leukocytes and the risk of ischemic diseases. Journal of the American Medical Association 257: 2318–2324, 1987
Evangelista V, Piccardoni P, de Gaetano G, Cerletti C. Defibrotide inhibits platelet activation by cathepsin G released from stimulated polymorphonuclear leukocytes. Thrombosis and Haemostasis 67 660–664, 1992
Fareed J, Kindel G, Walenga J. Pharmacologic validation of the antithrombotic and vascular effects of defibrotide. In Symposium on new evidence in the pathogenesis of POAD, role of new antithrombotic agents. Preprinted from the proceedings of the 15th world congress of the International Union of Angiology, pp (1–7), Elsevier Science Publishers, Amsterdam, 1989a
Fareed J, Walenga JM, Cornelli U. Antithrombotic drugs in pelvic surgery. Seminars in Thrombosis and Hemostasis 15: 230–232, 1989b
Fareed J, Walenga JM, Hoppensteadt DA, Kumar A, Ulutin ON, et al. Pharmacological profiling of defibrotide in experimental models. Seminars in Thrombosis and Hemostasis 14: 27–37. 1988
Ferrari A, Dindelli M, Sellaroli CM. Preventing postoperative deep venous thrombosis in gynecological surgery with defibrotide. International Surgery 75: 184–188, 1990
Ferrari P, Cornelli U, Dina F, Gerosa C, Nazzari M, et al. Defibrotide in the prevention of deep vein thrombosis in general surgery. Preliminary results of multicentre study. In Italian. Minerva Medica 79: 551–561, 1988
Ferrero ME, Marni A, Salari PC, Spaggiari PG, Gaja G, Usefulness of defibrotide treatment during organ procurement and preservation in rats. Transplantation Proceedings 23: 2359–2361, 1991
Frascà GM, Vangelista A, Raimondi C, Bonomini V. Prevention of vascular graft lesions in renal transplant recipients with a new antithrombotic agent (Defibrotide): a controlled study. Life Support Systems 4: 231–137, 1986
Fumagalli G, Angelaccio E, Lombardo N, Clementi F. Morphometric and ultrastructural study of experimental venous thrombosis. Effects of defibrotide, an antithrombotic agent. Haemostasis 17: 361–370, 1987
Gelister JSK, James WE, Fox JA. Preliminary experience with defibrotide in severe lower limb ischaemia. Haemostasis 16 (Suppl. 1): 63–66, 1986
Gensini G, Paniccia R, Prisco D, Francalanci I, Bandinelli B. Effect of defibrotide (DEF) on fibrinolysis (F) in patients with peripheral obstructive vascular disease (POVD). Abstract. Thrombosis and Haemostasis 65: 1784, 1991
Gerosa C, Calvani AB, Cornelli U, Dina F, Ferrari PA, Ratti G, et al. A multicentre study with defibrotide in the prophylaxis of deep vein thrombosis. In Italian. Minerva Chirurgica 44: 1507–151, 1989
Giambruno M, Caracciolo G, Lococo L, Abbadessa V, Caiozzo A, et al. Defibrotide in the treatment of acute thrombophlebitis: clinical efficacy and activity against the fibrinolytic system. In Italian. Giornale Italiano di Angiologia 8: 35–41, 1988
Gorman RR. Modulation of human platelet function by prostacyclin and thromboxane A2. Federation Proceedings 38: 83–88, 1979
Hohlfeld T, Strobach H, Schrör K. Stimulation of prostacyclin synthesis by defibrotide: improved contractile recovery from myocardial, stunning,. Journal of Cardiovascular Pharmacology 17: 108–115, 1991
Hohlfeld T, Thiemermann C, Schrör K. Protection from myocardial ischaemic injury in cats and pigs by defibrotide — different mode of action? Prostaglandins in Clinical Research: Cardiovascular System: 137-141, 1989
Interdonato F, Festi L, Gerosa C, Benevento A, Dionigi R. Prophylaxis of deep vein thrombosis after radical or modified mastectomy. In Italian. Minerva Chirurgica 45: 1389–1392, 1990
Jarrett PEM, Morland M, Browse NL. Treatment of Raynaud’s phenomenon by fibrinolytic enhancement. British Medical Journal 2: 523–525, 1978
Kuehl FA, Dougherty HW, Ham EA. Interactions between prostaglandins and leukotrienes. Biochemical Pharmacology 33: 1–5, 1984
La Russa A, Ghiringhelli P, Cauzzi L, Pecchini P, Gruttad’Auria C, et al. Continuous infusion of defibrotide in the prevention of thrombosis of the extracorporeal circuit during standard haemodialysis. In Italian. Terapia Moderna 5: 1–3, 1991
Lippi G, Viliani T, Troni GM, Boffi L, Doni A. Defibrotide versus heparin in the prophylaxis of deep vein thrombosis in general surger. Giornale Italiano di Angiologia 7: 152–157, 1987
Lippi G, Viliani T, Alessandrello-Liotta A, Morelli P, Zucconi G, et al. Defibrotide versus heparin in antithrombotic prophylaxis in gynaecological surgery. Minerva Medica 80: 455–459, 1988
Löbel P, Schrör K. Selective stimulation of coronary vascular PGI2 but not of platelet thromboxane formation by defibrotide in the platelet perfused heart. Naunyn-Schmiedeberg’s Archives of Pharmacology 331: 125–130, 1985
Löbel P and Schrör K. Stimulation of vascular prostacyclin and inhibition of platelet function by oral defibrotide in cholesterol-fed rabbits. Atherosclerosis 80: 69–79, 1989
Macchione C, Molaschi M, Neirotti M, Longo F, Ferrario E, et al. Hemodynamic, hemorheological and coagulative changes after treatment with defibrotide in elderly patients affected by chronic arteriopathy of the lower limbs. Archives of Gerontology and Geriatrics (Suppl. 2): 413–416, 1991
Marchetti M, Poggi S, Mandolesi S, Santoro P, Ricci S, et al. Assessing the therapeutic effectiveness of defibrotide administered parenterally, then orally, to patients with occlusive arteriopathy of the lower limbs (stage II and III of Leriche-Fontaine) a controlled study versus buflomedil. In Italian. Giornale Italiano di angiologia 8: 182–187, 1988
Marelli C, Belcaro G, Girardello R. Defibrotide in patients with intermittent claudication, improvement in blood flow, fibrinolytic activity and microcirculation after six months of treatment. Therapeutic Research 47: 459–45, 1990
Mattioli G, Cappello C, Fusaro MT. Treatment of acute myocardial infarction with defibrotide. Seminars in Thrombosis and Hemostasis 15: 470–473, 1989
Milazzotto F, Carelli M, Citone C, Di Marcotullio G, Giampaolo P, et al. Use of defibrotide in the treatment of acute myocardial infarction. Seminars in Thrombosis and Hemostasis 15: 464–469, 1989
Moser KM. Pulmonary embolism. In Wilson JD, Braunwald E, Isselbacher KJ, Petersdorf RG, Martin JB, et al. (Eds) Harrison’s principles of internal medicine, 12th ed., Vol 2, 12th edition: pp. 1092–1096, McGraw-Hill, Inc. Health Professionals Division, New York, 1991
Murgiano GA. Evaluation of the efficacy of profibrinolytic treatment with defibrotide in the therapy of thrombophlebitis. In Italian. La Clinica Terapeutica 127: 355–361, 1988
Natili G, Persia D, Agrati AM, Nazzari M. Defibrotide: an antithrombotic alternative to heparin in uraemic patients with high haemorrhagic risk subjected to haemodialysis. In Italian. Medical Praxis 11: 1–8, 1990
Ney P. Agonist-induced human neutrophil activation is inhibited by defibrotide in vitro. Naunyn-Schmiedeberg’s Archives of Pharmacology 341: R57, 1990
Niada R, Mantovani M, Prino G, Pescador R, Berti F, et al. Antithrombotic activity of a polydeoxyribonucleotide substance extracted from mammalian organs: a possible link with prostaglandin. Thrombosis Research 23: 233–246, 1981
Niada R, Porta R, Pescador R, Mantovani M, Prino G, et al. Cardioprotective effects of defibrotide in acute myocardial ischaemia in the cat. Thrombosis Research 38: 71–81, 1985
Niada R, Pescador R, Porta R, Mantovani M, Prino G. Defibrotide is antithrombotic and thrombolytic against rabbit venous thrombosis. Haemostasis 16 (Suppl. 1): 3–8, 1986
Niada R, Mantovani M, Prino G, Pescador R, Porta R. PGI2-generation and antithrombotic activity of orally administered defibrotide. Pharmacological Research Communications 14: 949–957, 1982
Noseda G, Fragiacomo C, Ferrari D. Pharmacokinetics of defibrotide in healthy volunteers. Haemostasis 16 (Suppl. 1): 26–30, 1986
Palareti G, Guazzaloca G, Legnani C, Leali N, Busacchi P, et al. Prophylaxis of venous thrombosis after gynaecological surgery. A controlled pilot study of defibrotide. Haematologica 77: 44–48, 1992
Patrassi GM, Sartori MT, Viero ML, Scapinello MP, Boeri G, et al. Fibrinolytic effects of defibrotide in atherosclerotic patients. Seminars in Thrombosis and Hemostasis 17 (Suppl. 1): 101–105, 1991
Perico N, Schieppati A, Mecca G, Rossi EC, Remuzzi G. Prostacyclin and renal diseases. Clinical Nephrology 18: 111–119, 1982
Pescador R, Mantovani M, Prino G. Pharmacokinetics of defibrotide and of its profibrinolytic activity in the rabbit. Thrombosis Research 30: 1–11, 1983
Pescador R, Porta R, Conz A, Mantovani M, Prino G. Defibrotide decreases cholesterol amount in hypercholesterolemic rabbit aorta, with no modification of plasma or lipoprotein cholesterol. Life Sciences 44: 789–797, 1989
Pogliani EM, Salvatore M, Fowst C, Marelli C. Bioequivalence study of two dosage schemes for defibrotide on the parameters of fibrinolysis in healthy volunteers. In Italian. Farmica and Terapia 4: 5, 1987
Pogliani EM, Salvatore M, Fowst C, Girardello R, Marelli C. Effects of a defibrotide — heparin combination on some measures of haemostasis in healthy volunteers. Journal of International Medical Research 17: 36–40, 1989
Pola P, Simeoni M, Favuzzi A, Gasbarri V. A new approach to the pharmacological therapy of claudication: preliminary data. In Italian. Giornale Italiano di angiologia 8: 28–32, 1988
Porta R, Pescador R, Mantovani M, Niada R, Prino G, et al. Pharmacokinetics of defibrotide and of its profibrinolytic activity in different animal species. Effects on the levels of fibrinolysis inhibitors and of fibrinogen/fibrin degradation products (FDP). 7th International Congress on Fibrinolysis, March 1984. Abstract, pp. (322–326), 1984
Porta R, Moltrasio D, Pescador R, Lanzarotti E, Prino G. High-performance liquid chromatography determination of polydeoxyribonucleotides in plasma: its application to the determination of defibrotide’s pharmacokinetics in the rabbit. Analytical Biochemistry 204: 143–146, 1992
Porta R, Pescador R, Mantovani M, Prino G. Defibrotide affects the anticoagulant and lipase-releasing activities of heparin. Haemostasis 20: 347–356, 1990
Prino G, Mantovani M, Niada R. Antithrombotic activity of a polydesoxynucleotidic-like substance (Faction P). Advances in coagulation, fibrinolysis, platelet aggregation and atherosclerosis, Palermo, October 6-9, 1976, pp. (282–289), 1976
Ranieri G, De Mitrio V, Petronelli M, Ancona A, Dammacco F. Using defibrotide for treating the Raynaud phenomenon associated with progressive systemic sclerosis or essential mixed cryoglobulinemia. In Italian. Clinica Terapeutica 126: 335–343, 1988
Rizzi A, Radaelli F, Pisano M, Orro S, Nazzari M, et al. Defibrotide for the prophylaxis of deep venous thrombosis in thoracic surgery: a controlled multicenter study versus heparin. In Italian. Minerva Medica 78: 745–750, 1987
Romeo S, Bonaccorsi R, Iapichino G, Viola S, Carullo F. Defibrotide in peripheral obstructive arteriopathy: a multicenter study. In Italian. Medical Praxis 10: 141–149, 1989
Rossi R, Farma A, Minoretti C, Martinelli D, Colantonio G, et al. Defibrotide: an alternative to heparin in blood dialysis. In Italian. Giornale Italiano Di Nefrologia 5: 125–130, 1988
Rossi R, Farma A, Maggi GC, Marelli A. Defibrotide pharmacokinetics in uremic patients in dialysis. In Italian. Minerva Medica 82: 853–857, 1991
Sabbá C, Zupo V, Dina F, Nazzari M, Albano O. A pilot evaluation of the effect of defibrotide in patients affected by peripheral arterial occlusive disease. International Journal of Clinical Pharmacology, Therapy and Toxicology 26: 249–252, 1988
Salavdori M, Mondaini F, Martinelli F, Filimberti E, Cinotti S, et al. Use of defibrotide in the haemodialytic treatment of patients at high risk of haemorrhage: preliminary experience. In Italian. Giornale Italiano Di Ricerche Cliniche E Terapeutiche 11: 36–40, 1990
Schrör K, Addicks K, Darius H, Ohlendorf, Rösen P. PGI2 inhibits ischemia-induced platelet activation and prevents myocardial damage by inhibition of catecholamine release from adrenergic nerve terminals. Evidence for cAMP as common denominator. Thrombosis Research 21: 175–180, 1981.
Schrör K, Ackermann G, Hohlfeld T, Ney P, Schröder H, Strobach H. Endothelial protection by defibrotide — a new strategy for treatment of myocardial infarction? Zeitschrift für Kardiologie 78 (Suppl. 6): 35–41, 1989a
Schrör K, Thiemermann C, Löbel P. Stimulation of vascular prostacyclin formation by defibrotide: a new strategy for treatment of acute myocardial ischaemia. In Schmutzler H, Rutsch W, Dougherty FC (Eds) Limitation of infarct size, pp. 83–93, Springer-Verlag, Berlin, 1989b
Schwab SJ, Onorato JJ, Sharar LR, Dennis PA. Hemodialysis without anticoagulation. One-year prospective trial in hospitalized patients at risk for bleeding. American Journal of Medicine 83: 405–410, 1987
Sciacca V, Chirletti P, Cisternino S, Apice N, Bononi M, et al. Defibrotide versus calcium heparin in DVT prophylaxis in abdominal surgery. In Italian. Chirurgia 4: 486–488, 1991
Scott G, Fisher J, Johnston AM, Pescador R. Disposition studies with [125I]-defibrotide. Abstract. European Society of Biochemical Pharmacology. XII European Workshop on Drug Metabolism, Basel, Switzerland, September 16–21, 1990. Abstract P4.55, p. 179, 1990
Seccia M, Bortolotti P, Bellomini M, Buccianti P, Chiarugi M et al. Use of defibrotide in the treatment of acute superficial thrombophlebitis of the limbs. In Italian. Minerva Chirurgica 44: 1379–1384, 1989
Simpson P, Mickelson J, Fantone JC, Gallagher KP, Lucchesi BR. Reduction of experimental canine infarction size with prostaglandin E1: inhibition of neutrophil migration and activation. Journal of Pharmacology and Experimental Therapeutics 244: 619–624, 1988
Spinella G, Nazzari M. Study of the therapeutic efficacy of defibrotide during acute thrombophlebitis. In Italian. Giornale Italiano di Ricerche Clinche e Terapeutische 8: 71–74, 1987
Spinella G, Martino A, Nazzari M. Effectiveness of oral and parenteral treatment with defibrotide in chronic peripheral obstructive arterial disease. In Italian, Farmaci 12: 135–140, 1988
Strano A, Fareed J, Sabbá C, Albano O, Allegra C, et al. A double-blind, multicenter, placebo-controlled, dose comparison study of orally administered defibrotide: preliminary results in patients with peripheral arterial disease. Seminars in Thrombosis and Hemostasis 17 (Suppl. 2): 228–234, 1991
Swartz RD, Port FK. Preventing hemorrhage in high-risk hemodialysis: regional versus low-dose heparin. Kidney International 16: 513–518, 1979
Thiemermann C, Löbel P, Schrör K. Usefulness of defibrotide in protecting ischemic myocardium from early reperfusion damage. American Journal of Cardiology 56: 978–982, 1985
Thiemermann C, Thomas GR, Vane J.R. Defibrotide reduces infarct size in a rabbit model of experimental myocardial ischaemia and reperfusion, and regional myocardial blood flow on the ischaemic reperfused rabbit heart. British Journal of Pharmacology 97: 401–408, 1989
Tubaro M, Mattioli G, Cappello C, Natale E, Gerardi P, et al. Comparison of defibrotide vs heparin in preventing early rethrombosis after urokinase thrombolytic therapy in acute myocardial infarction. Cardiovascular Drugs and Therapy 5: Abstract P189, 1991
Ulutin ON. Clinical effectiveness of defibrotide in vaso-occlusive disorders and its mode of actions. Seminars in Thrombosis and Hemostasis 14 (Suppl.): 58–63, 1988
Ulutin ON, Ilhan-Berkel N, Tunali H, Özer M, Balkuv-Ulutin S, et al. Effects of defibrotide on peripheral obliterative vascular diseases. Haemostasis 16 (Suppl.): 59–62, 1986
Varvara M, Leone MA, Iacobellis MA, Claudatus J, Manno C. Long term experience of the substitution of heparin with defibrotide in haemodialysis patients. In Italian. Medical Praxis 11: 1–5, 1990
Ventura R, Pastore U, Attolini R, Pietrogrande V, Gerosa C. Prevention of deep vein thrombosis after orthopaedic surgery. In Italian. Ortopedia e Traumatologia Oggi 11: 137–138, 1991
Verstraete M, Boogaerts M. Haematological disorders. In Speight TM (Ed.) Avery’s Drug Treatment. Principles and practice of clinical pharmacology and therapeutics, 3rd ed., Adis Press, Auckland, pp. 958–1022, 1987
Viano L, Colangelo D, Silvestro L, Da Col R, Nazzari M, et al. The pharmacokinetics of defibrotide (125-1) in humans. In Italian. Medical Praxis 13: 1–14, 1992
Villani LG, Dalla Valle R, Rubini P. Defibrotide and heparin in the prophylaxis of deep vein thrombosis. A controlled study. In Italian. Minerva Chirurgica 45: 1029–1033, 1990
Violi F, Ferro D, Saliola M, Quintarelli C, Basili S, et al. Effect of oral defibrotide on tissue-plasminogen activator and tissue-plasminogen activator inhibitor balance. European Journal of Clinical Pharmacology 42: 379–383, 1992
Violi F, Ferro D, Alessandri C, Quintarelli C, Saliola M, et al. Inhibition of tissue plasminogen activator inhibitor by defibrotide in atherosclerotic patients. Seminars in Thrombosis and Hemostasis 15: 226–229, 1989
Vittoria A, Messa GL, Berri F, De Giovanni F, Buzzetti G, et al. Effect of defibrotide administered I.V., I.M. and per os on some fibrinolytic parameters in man. Controlled study versus placebo. In Davidson JF, Donati MB, Coccheri S (Eds) Progress in fibrinolysis 6, Churchill Livingstone, Vol. 7, pp. 329–335, 1985
Walsh JJ, Bonnar J, Wright FW. A study of pulmonary embolism and deep leg vein thrombosis after major gynaecological surgery using labelled fibrinogen-phlebography and lung scanning. The Journal of Obstetrics and Gynaecology of the British Commonwealth. 81: 311–316, 1974
Weichert W, Breddin HK, Staubesand J. Application of a laser-induced endothelial injury model in the screening of antithrombotic drugs. Seminars in Thrombosis and Hemostasis 14 (Suppl.): 106–114, 1988
Werns SW, Lucchesi BR. Inflammation and myocardial infarction. British Medical Bulletin 43: 460–471, 1987
Ygge J. Changes in blood coagulation and fibrinolysis during the postoperative period. American Journal of Surgery 119: 225–232, 1970
Zanasi R, Fioretta G, Ciocia G, Bergonzi M. Prevention of deep venous thrombosis in orthopedic surgery: effects of defibrotide. Clinical Therapeutics 10: 350–357, 1988
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: S. Coccheri, Servizio di Angiologia e Malattie della Coagulazione, Bologna, Italy; J.D. Coffman, Vascular Medicine Section, The University Hospital, Boston, Massachusetts, USA; T. Di Perri, Università degli Studi di Siena, Istituto di Clinica Medica Generale e Terapia Medica, Siena, Italy; J. Fareed, Hemostasis and Thrombosis Research Laboratories, Loyola University, Chicago, Illinois, USA; T. Hohlfeld, Institut für Pharmakologie, Medizinische Einrichtungen der Universität Düsseldorf, Düsseldorf, Federal Republic of Germany; M. Rozenberg, Department of Haematology, The Prince of Wales Hospital, Randwick, New South Wales, Australia; C. Thiemermann, The William Harvey Research Institute, St Bartholomew’s Hospital Medical College, London, England; A.J. Tiefenbrunn, Department of Internal Medicine, Washington University School of Medicine, St Louis, Missouri, USA; O.N. Ulutin, Division of Haematology and Haemostasis and Thrombosis Research Center, Department of Medicine, Cerrahpasa Medical School of Istanbul University, Istanbul, Turkey; M. Verstraete, Center for Thrombosis and Vascular Research, University of Leuven, Leuven, Belgium.
Rights and permissions
About this article
Cite this article
Palmer, K.J., Goa, K.L. Defibrotide. Drugs 45, 259–294 (1993). https://doi.org/10.2165/00003495-199345020-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199345020-00007