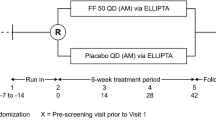
Abstract
Synopsis
Fluticasone propionate is an androstane carbothioate glucocorticosteroid with almost twice the topical anti-inflammatory potency of beclomethasone dipropionate. Importantly, it is not appreciably absorbed from. the gastrointestinal tract. However, the fraction of active drug absorbed from the lungs after inhalation, and therefore total systemic availability, has yet to be determined.
Inhaled fluticasone propionate administered at dosages of 1500 μg/day for 1 year or 2000 μg/day for 6 weeks did not cause clinically significant pituitary-adrenal suppression. Preliminary data from 2 published trials also indicate no significant effect on growth in children. However, wider clinical experience is needed to clarify the effects of long term administration on pituitaryadrenal function, bone metabolism and attainment of adult height in children.
In clinical studies, inhaled fluticasone propionate was at least as effective as beclomethasone dipropionate or budesonide when administered at half the dosage of the comparators in patients with mild to moderate or severe asthma. Limited data suggest that fluticasone propionate also has considerable potential in the management of childhood asthma. In trials of up to I year in duration, fluticasone propionate appeared to be well tolerated by both adults and children.
Whether an improved tolerability profile compared with other corticosteroids is a major clinical benefit of the extremely low oral bioavailability of inhaled fluticasone propionate requires confirmation. Nevertheless, on the basis of available data from initial clinical trials of mostly limited duration, inhaled fluticasone propionate offers an effective treatment option for the management of asthma, with the potential of an enhanced safety profile.
Pharmacological Properties
Relative topical vasoconstrictor potency values (arbitrary units) in humans were considerably greater for fluticasone propionate (945) than for fluocinolone acetonide (100) or beclomethasone dipropionate (50). In an animal model, an index of topical to systemic activity determined for fluticasone propionate (91) was superior to that for fluocinolone acetonide (1) and beclomethasone dipropionate (0.4).
In vitro, the affinity of fluticasone propionate for the glucocorticoid receptor of human lung cytosol exceeded that of budesonide, dexamethasone and flunisolide. Preliminary data suggest that long term fluticasone propionate therapy may reduce bronchial hyper-reactivity and airways inflammation.
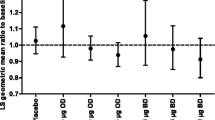
Single-dose oral fluticasone propionate 16mg or intranasal fluticasone propionate 4000 μg/day administered for 7 days had no significant effect on mean morning plasma cortisol levels in healthy volunteers. Similarly, no significant reductions in morning plasma or serum cortisol levels were demonstrated in adult asthma patients treated with inhaled fluticasone propionate at dosages of 1500 μg/day for 1 year and 2000 μg/day for 6 weeks, or in asthmatic children administered dosages of 100 or 200 μg/day for up to 9 months. Furthermore, initial comparative studies suggest that inhaled fluticasone propionate may have less effect on pituitary-adrenal function in adult and paediatric patients, and growth of long bones in children, than beclomethasone dipropionate.
Systemic effects of inhaled corticosteroids are due to the absorption of active drug from the gastrointestinal and lower respiratory tracts. Preliminary pharmacokinetic data indicate that the oral bioavailability of fluticasone propionate is negligible. However, the fraction of unchanged drug absorbed from the lungs after inhalation has not been determined and thus the overall systemic availability of inhaled fluticasone propionate is unknown.
Therapeutic Efficacy
The efficacy of inhaled fluticasone propionate 50 to 2000 μg/day administered for up to 1 year has been assessed in more than 4000 patients with asthma.
In the treatment of mild to moderate asthma, inhaled fluticasone propionate 50 to 200 μg/day was more effective than placebo as shown by significant improvements in lung function and symptom scores, and reduced use of supplementary anti-asthmatic medication.
Comparisons with active agents have shown that fluticasone propionate 200 to 1000 μg/day is at least as effective as beclomethasone dipropionate 400 to 2000 μg/day in the treatment of asthma, including severe disease. Fluticasone propionate 200 and 1000 μg/day was significantly more effective than budesonide 400 and 1600 μg/day, respectively. In patients with moderate asthma, fluticasone propionate 500 μg/day was significantly more effective than nedocromil 16 mg/day. Fluticasone propionate 1500 or 2000 μg/day has also facilitated the discontinuation of oral prednisone in patients with severe asthma.
Limited data have demonstrated the usefulness of fluticasone propionate in the treatment of childhood asthma. Fluticasone propionate 200 μg/day was at least as effective as beclomethasone dipropionate 400 μg/day, while a dosage of 100 μg/day was significantly more effective than sodium cromoglycate 80 mg/day.
Tolerability
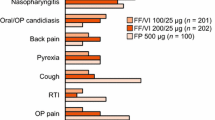
Clinical studies suggest that treatment with inhaled fluticasone propionate for up to 1 year is well tolerated by adults at dosages of up to 2000 μg/day and by paediatric patients at dosages of up to 200 μg/day. The most commonly reported adverse events included oral candidiasis, dysphonia and upper respiratory tract infection. However, data on longer term treatment are required, particularly in children.
Fluticasone propionate 100 to 1500 μg/day was as well tolerated as beclomethasone dipropionate 400 to 2000 μg/day, with both drugs producing a similar spectrum of local adverse events. Fluticasone propionate administered at dosages of 100, 500 and 2000 μg/day, respectively, demonstrated equivalent tolerability to sodium cromoglycate 80 mg/day, nedocromil 16 mg/day and budesonide 1600 μLg/day.
Dosage and Administration
Twice-daily inhaled fluticasone propionate at total daily dosages of 50 to 2000μg has been used for the treatment of mild to severe asthma in adults, while dosages of 100 and 200 μg/day have been used in childhood asthma. Inhaled fluticasone propionate is available as metered dose aerosol and dry powder formulations.
Similar content being viewed by others
References
Al-Habet S, Rogers HJ. Pharmacokinetics of intravenous and oral prednisolone. British Journal of Clinical Pharmacology 10: 503–508, 1980
Ayres JG, Harris TAJ, Bateman ED, Lundback B. The effects of fluticasone propionate lmg and 2mg daily and budesonide 1.6mg daily in severe asthmatics. Abstract. Thorax, in press, 1994
Barnes NC, Marone G, Di Maria GU, Visser S, Utama I, et al. A comparison of fluticasone propionate lmg daily with beclomethasone dipropionate 2mg daily in the treatment of severe asthma. European Respiratory Journal 6: 877–884, 1993
Barnes PJ, Lee TH. Recent advances in asthma. Postgraduate Medical Journal 68: 942–953, 1992
Booth H, Gardiner PV, Ward C, Walls A, Hendrick DJ, et al. Effect of fluticasone propionate on airway inflammation as assessed by bronchoalveolar lavage (BAL). Abstract PI867. European Respiratory Journal 6 (Suppl. 17): 584, 1993
British Thoracic Society. Guidelines for management of asthma in adults. I. Chronic persistent asthma. British Medical Journal 301: 651–653, 1990
Bryson HM, Faulds D. Intranasal fluticasone propionate. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in allergic rhinitis. Drugs 43: 760–775, 1992
Chaplin MD, Rooks II W, Swenson EW, Cooper WC, Nerenberg C, et al. Flunisolide metabolism and dynamics of a metabolite. Clinical Pharmacology and Therapeutics 27: 402–413, 1980
Chervinsky P, Bronsky E, Dockhorn R, LaForce C, Noonan M, et al. Fluticasone propionate aerosol in asthma. Abstract 464. Journal of Allergy and Clinical Immunology 87: 255, 1991
Dahl R. The safety and efficacy of fluticasone propionate 200μg daily given via the Diskhaler inhaler as compared with the pressurised metered dose inhaler in adult asthma. Abstract P36. Clinical and Experimental Allergy 23 (Suppl. 1): 81, 1993a
Dahl R, Lundback B, Malo J-L, Mazza JA, Markku M, et al. A dose-ranging study of fluticasone propionate in adult patients with moderate asthma. Chest 104: 1352–1358, 1993b
Duggan DE, Yeh KC, Matalia N, Ditzler CA, McMahon FG. Bioavailability of oral dexamethasone. Clinical Pharmacology and Therapeutics 18: 205–209, 1975
Fabbri L, Burge PS, Croonenborgh L, Warlies F, Weeke B, et al. Comparison of fluticasone propionate with beclomethasone dipropionate in moderate to severe asthma treated for one year. Thorax 48: 817–823, 1993
Ganong WF. Review of Medical Physiology, 10th ed., Lange Medical Publications, Los Altos, 1981
Geddes DM. Inhaled corticosteroids: benefits and risks. Thorax 47: 404–407, 1992
Götz M. The safety and efficacy of inhaled fluticasone propionate in childhood asthma. Abstract 804. European Respiratory Journal 4 (Suppl. 14): 404, 1991
Gustafsson P, Tsanakas J, Gold M, Primhak R, Radford M, et al. Comparison of the efficacy and safety of inhaled fluticasone propionate 200 μg/day with inhaled beclomethasone dipropionate 400 μg/day in mild and moderate asthma. Archives of Disease in Childhood 69: 206–221, 1993
Harding SM. The human pharmacology of fluticasone propionate. Respiratory Medicine 84 (Suppl. A): 25–29, 1990
Hawthorne AB, Record CO, Holdsworth CD, Giaffer MH, Burke DA, et al. Double blind trial of oral fluticasone propionate v prednisolone in the treatment of active ulcerative colitis. Gut 34: 125–128, 1993
Hoffmann-Streb A, L’Allemand D, Buettner-Goetz P, Wahn U. Adrenal function in asthmatic children treated with fluticasone or budesonide. Abstract 688. Journal of Allergy and Clinical Immunology 87 (Suppl. 1): 311, 1991
Högger P, Rawert I, Rohdewald P. Dissolution, tissue binding and kinetics of receptor binding of inhaled glucocorticoids. Abstract P1864. European Respiratory Journal 6 (Suppl. 17): 584, 1993
International Consensus Report on Diagnosis and Treatment of Asthma. European Respiratory Journal 5: 601–641, 1992
Kellerman D, van As A, O’Connell MB, Nathan R, Baker JS, et al. The role of pulmonary function testing for detecting dose response relationships of inhaled corticosteroids in asthma. Abstract. American Review of Respiratory Diseases 143 (Suppl. 4): 625, 1991
Kerrebijn KF. Paediatric asthma, prospects for control or cure. Clinical and Experimental Allergy 21 (Suppl. 1): 93–104, 1991
Kerrebijn KF. Use of topical corticosteroids in the treatment of childhood asthma. American Review of Respiratory Disease 141: 77–81, 1990
Langdon CG, Thompson J. Fluticasone propionate: a comparison with budesonide in adult asthmatic patients. Abstract 1232. European Respiratory Journal 5 (Suppl. 15): 368, 1992
Larsen GL. Asthma in children. New England Journal of Medicine 326: 1540–1545, 1992
Leblanc P, Mink S, Keistinen T, Saarelainen PA, Ringdal N, et al A comparison of fluticasone propionate 200 μg/day with beclomethasone dipropionate 400 μg/day in adult asthma. Allergy, in press, 1994
Lozewicz S, Wang J, Duddle J, Thomas K, Chalstrey S, et al. Topical steroids inhibit activation by allergen in the upper respiratory tract. Journal of Allergy and Clinical Immunology 89: 951–957, 1992
Lundback B, Alexander M, Day J, Hebert J, Holzer R, et al. Evaluation of fluticasone propionate (500μg day−1) administered either as dry powder via a Diskhaler® inhaler or pressurised inhaler and compared with beclomethasone dipropionate (1000μg day®) administered by pressurised inhaler. Respiratory Medicine 87: 609–620, 1993
MacKenzie CA, Wales JKH. Clinical experience with inhaled fluticasone propionate — childhood growth. Abstract 0621. European Respiratory Journal 6 (Suppl. 17): 262, 1993
MacKenzie CA, Weinberg EG, Tabachnik E, Taylor M, Havnen J, et al. A placebo controlled trial of fluticasone propionate in asthmatic children. European Journal of Pediatrics 152: 856–860, 1993
Meltzer EO, Orgel HA, Bronsky EA, Furukawa CT, Grossman J, et al. A dose-ranging study of fluticasone propionate aqueous nasal spray for seasonal allergic rhinitis assessed by symptoms, rhinomanometry, and nasal cytology. Journal of Allergy and Clinical Immunology 86: 221–230, 1990
Nathan RA, Bronsky EA, Fireman P, Grossman J, LaForce CF, et al. Once daily fluticasone propionate aqueous nasal spray is an effective treatment for seasonal allergic rhinitis. Annals of Allergy 67: 332–338, 1991
Noonan MJ, Chervinsky P, Weisberg SC, Busse WW, Cook CK, et al. Fluticasone propionate (FP) aerosol in the treatment of oral corticosteroid dependent asthmatics. Abstract P1871. European Respiratory Journal 6 (Suppl. 17): 585, 1993
Pauli G, Dietemann A, Desfougères JL. Fluticasone propionate is more effective and as safe as nedocromil in the treatment of moderate asthma. Abstract P1872. European Respiratory Journal 6 (Suppl. 17): 586, 1993a
Pauli G, Dietemann A, Desfougères JL. Fluticasone propionate is more effective and as safe as nedocromil in the treatment of moderate asthma. Poster. European Respiratory Society Congress, Florence, Sep 25–29, 1993b
Phillipps GH. Structure-activity relationships of topically active steroids: the selection of fluticasone propionate. Respiratory Medicine 84 (Suppl. A): 19–23, 1990
Price JF. Comparative data in childhood asthma. Abstract 1090. European Respiratory Journal 5 (Suppl. 15): 326, 1992
Rehder S, Wurthwein G, Rohdewald P. Fluticasone propionate, a topically applied glucocorticoid with high intrinsic activity. Abstract 932. European Respiratory Journal 4 (Suppl. 14): 444, 1991
Ryrfeldt Å, Andersson P, Edsbäcker S, Tönnesson M, Davies D, et al. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. European Journal of Respiratory Diseases 63 (Suppl. 122): 86–95, 1982
Schleimer RP. The mechanisms of antiinflammatory steroid action in allergic diseases. Annual Review of Pharmacology and Toxicology 25: 381–412, 1985
Sheffer AL, LaForce C, Schoenwetter W, Seiner J, Pearlman D, et al. Fluticasone propionate (FP), a new potent inhaled corticosteroid, in steroid-naive patients. Abstract P1870. European Respiratory Journal 6 (Suppl. 17): 585, 1993
Tattersfield AE, Barnes PJ. β2-Agonists and corticosteroids: new developments and controversies. Report of a Meeting 1990. American Review of Respiratory Disease 146: 1637–1641, 1992
Thomas KE, Greenwood L, Murrant N, Cook J, Devalia L, et al. The effects of topical fluticasone propionate on allergen-induced immediate nasal airways response and eosinophil activation: preliminary results. Respiratory Medicine 84 (Suppl. A): 33–35, 1990
Toogood JH. Complications of topical steroid therapy for asthma. American Review of Respiratory Disease 141 (Suppl.): 89–96, 1990
van As A, Kellerman D, Rogenes PR, Kral K. Assessing duration of action of inhaled corticosteroids in asthma. Abstract 934. European Respiratory Journal 4 (Suppl. 14): 445, 1991
Wolthers OD, Pedersen S. Short term growth during treatment with inhaled fluticasone propionate and beclomethasone dipropionate. Archives of Disease in Childhood 68: 673–676, 1993
Zarkovic J, Götz M. Fluticasone propionate-induced modulation of bronchial hyperreactivity of asthmatics. Abstract 1274. European Respiratory Journal 4 (Suppl. 14): 546, 1991
Zarkovic J, Götz M. Fluticasone propionate and bronchial hyperreactivity. Abstract 0582. European Respiratory Journal 5 (Suppl. 15): 181, 1992
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: P.J. Barnes, Department of Thoracic Medicine, Royal Brompton Hospital, London, England; K. Bauer, Department of Clinical Pharmacology, University of Vienna Medical School, Vienna, Austria; J. Dolovich, Department of Pediatrics, McMaster University, Hamilton, Ontario, Canada; M. Götz, Magistrat der Stadt Wien, Allgemeines Krankenhaus, Wien, Austria; C.A. MacKenzie, Department of Paediatrics, Sheffield Children’s Hospital, Sheffield, England; H. Malmberg, Department of ENT, University Central Hospital, Helsinki, Finland; B. Pedersen, Department of Respiratory Diseases, University Hospital of Aarhus, Aarhus, Denmark; A. Woodcock, South Manchester Health Authority, Wythenshawe Hospital, University School of Medicine, Manchester, England; J. Zarkovic, Magistrat der Stadt Wien, Allgemeines Krankenhaus, Wien, Austria.
Rights and permissions
About this article
Cite this article
Holliday, S.M., Faulds, D. & Sorkin, E.M. Inhaled Fluticasone Propionate. Drugs 47, 318–331 (1994). https://doi.org/10.2165/00003495-199447020-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199447020-00007