Abstract
Synopsis
Alteplase (recombinant tissue-type plasminogen activator) stimulates the fibrinolysis of blood clots by converting plasminogen to plasmin.
The efficacy of intravenous alteplase in the early treatment of patients with acute myocardial infarction has been unequivocally proven, and recent results from the GUSTO trial indicate a significant advantage in 30-day survival for alteplase in an accelerated dosage regimen (≤100mg infused over 90 minutes rather than 3 hours) over streptokinase. The advantage of the accelerated alteplase dosage regimen seems to be maintained for at least 1 year. The role of heparin as adjunctive therapy to thrombolysis remains to be fully defined but heparin administration appears to be more important in conjunction with alteplase than with streptokinase.
Ideally, patients should receive alteplase as soon as possible after the onset of symptoms of acute myocardial infarction and, while therapy is most beneficial when administered early, survival is improved when the drug is administered up to 12 hours after symptom onset. The accelerated regimen of alteplase used in the GUSTO trial demonstrated a survival advantage in patients ≤ 75 as well as those > 75 years of age which was at least as great as that seen with streptokinase. Similarly, alteplase reduces mortality in patients with both anterior and inferior infarctions; however, those with anterior wall infarctions show an improved outcome over those with inferior infarcts.
On the basis of pharmacoeconomic analysis of GUSTO data, the accelerated alteplase regimen cost an estimated additional $US32 678 per year of life saved compared with a conventional streptokinase regimen. Cumulative 1-year costs were greater in patients who received the accelerated alteplase regimen but survival was significantly greater than in patients who received streptokinase. No difference in quality of life was evident in patients who received either treatment.
The incidence of major haemorrhage associated with alteplase therapy appears to be similar to that seen with other fibrinolytic agents, increasing with increasing dose; however, the risk of stroke, particularly haemorrhagic stroke, is higher with alteplase than with streptokinase.
Thus, alteplase has become firmly established as a first-line option in the management of acute myocardial infarction. On the basis of accumulated evidence, the greatest risk reduction with alteplase therapy may be in certain high risk groups, such as those with anterior infarcts, selected elderly patients and those who present late after symptom onset.
Overview of Pharmacodynamic Properties
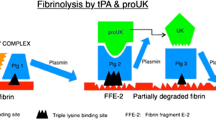
Alteplase is a serine protease, produced by recombinant DNA technology, which is chemically identical to human endogenous tissue-type plasminogen activator. Fibrinolysis of blood clots is stimulated when alteplase converts inactive endogenous plasminogen to plasmin in a manner similar to that of endogenous tissue-type plasminogen activator. Plasmin subsequently causes the breakdown of insoluble fibrin. Alteplase is relatively fibrin-specific (its activity is increased in the presence of fibrin), resulting in less depletion of plasma fibrinogen and fewer degradation products in the plasma of treated individuals than is seen with streptokinase, urokinase or saruplase.
The thrombolytic activity of alteplase has been adequately demonstrated in animal models of thrombosis, and the drug has been used clinically for several years. Alteplase has been associated with rebound antifibrinolytic effects possibly caused by potentiation of platelet aggregation which may be a partial cause of reocclusion in successfully treated patients.
Overview of Pharmacokinetic Properties
Because of its large molecular size, alteplase cannot easily diffuse across biological membranes and must be administered parenterally, usually intravenously.
After therapeutic doses of alteplase 90 to 100mg a maximal plasma concentration (Cmax) of 3 to 4 mg/L is achieved. When alteplase was administered in an accelerated regimen (90-minute infusion), steady-state alteplase concentrations (Css) for the initial infusion period were 45% higher than those for the standard administration protocol. Volume of distribution determinations for the central compartment ranged from 2.8 to 4.6L and were approximately one-half the volume of distribution at steady state.
Alteplase is cleared rapidly from plasma by the liver, with more than 50% removed within 5 minutes of drug administration. The rapid disappearance was most often described by use of a biexponential equation representing a 2-compartment model. The half-life associated with the initial phase of biphasic elimination in healthy individuals and patients with acute myocardial infarction ranged from 3.5 to 4.4 minutes. The area under the alteplase plasma concentration versus time curve (AUC) for the dominant initial phase of elimination accounted for 85 to 88% of the total AUC. Values for the terminal elimination half-life (t½β) were between 39 and 72 minutes. Total body clearance in healthy volunteers and most patients with acute myocardial infarction ranged from 34.3 to 38.4 L/h. Data regarding the pharmacokinetic profile of alteplase following an accelerated dosage regimen are limited but, other than a higher Css for the initial infusion period, appeared to be consistent with the profile reported for the original administration format.
Therapeutic Efficacy
The efficacy of alteplase in improving survival of patients with acute myocardial infarction is unequivocal. The benefit of adding aspirin to thrombolytic treatment is now firmly established, and the use of intravenous heparin in combination with alteplase has become widely accepted in clinical practice, despite a lack of definitive conclusions regarding its importance.
Improvements in various indices of left ventricular function, or differences in the degree of improvement, have been difficult to demonstrate consistently when comparing alteplase with other thrombolytic agents. On the basis of angiographic comparisons, patency was re-established more rapidly after administration of alteplase than after streptokinase, anistreplase or urokinase, although a ‘catch-up’ phenomenon resulted in virtually no difference between groups after the first few hours. Despite the theoretical associations between patency, left ventricular function and mortality, no survival advantage for any regimen was shown in the GISSI-2/ISG or ISIS-3 trials which compared alteplase (or duteplase) with streptokinase or anistreplase. However, the GUSTO trial showed a statistically robust (p ≤ 0.04) advantage in terms of reduced mortality at both 24 hours and 30 days for alteplase in an accelerated regimen (≤ 100 mg/90 min) plus intravenous heparin (30-day mortality rate 6.3% compared with 7.4% for streptokinase plus intravenous heparin, 7.2% for streptokinase plus subcutaneous heparin and 7% for the combination of alteplase and streptokinase plus intravenous heparin). In addition, the combined end-points of 30-day mortality and nonfatal stroke, nonfatal nonhaemorrhagic stroke or nonfatal disabling stroke also showed a significant (p ≤ 0.006) benefit in clinical outcome in patients who received the accelerated alteplase regimen compared with those in the streptokinase groups. A preliminary report indicates that the survival advantage of alteplase has persisted for at least 1 year.
Paradoxically, the achievement of more rapid coronary artery patency with alteplase than streptokinase and urokinase may actually provide coronary arteries with more opportunity to reocclude and, thus, may explain the higher reocclusion rates seen with alteplase in some trials. However, in the GUSTO angiographic study, no significant difference in reocclusion rates was reported between the 4 treatment groups on the basis of thrombolytic agent or patency. Although the combination of alteplase and streptokinase or urokinase produced lower reocclusion rates, no significant reduction in mortality resulted from this approach or from combining alteplase with percutaneous transluminal coronary angioplasty.
Several factors may be clinically useful predictors of patient outcome after an acute myocardial infarction and may help determine the benefit-to-risk ratio for the use of thrombolytic therapy. In general, there is a higher mortality rate associated with an anterior than an inferior infarction; thus, patients with anterior infarcts are likely to benefit more from aggressive thrombolytic therapy. The accelerated alteplase regimen used in the GUSTO trial reduced mortality in patients with both anterior and inferior infarcts compared with streptokinase; however, the magnitude of benefit was greater in those with anterior infarcts.
Increasing age is recognised as an independent risk factor in patients who experience acute myocardial infarction. In the GUSTO trial, subgroup analysis indicated that the accelerated alteplase regimen was at least as effective as streptokinase in patients both ≤ 75 or > 75 years of age and preliminary data on the basis of further age stratification suggested that alteplase was as effective in patients up to the age of 85 years.
Historically, thrombolytic therapy was reserved for patients receiving treatment 4 to 6 hours from symptom onset. The degree of benefit from alteplase, as with other thrombolytics, is clearly time-dependent; however, recent trial data, particularly from the LATE study, provided convincing evidence for a survival advantage using alteplase up to 12 hours after the onset of symptoms.
The cost-effectiveness value of using the accelerated alteplase regimen rather than the conventional streptokinase regimen in the GUSTO trial has been calculated to be $US32 678 per year of life saved. Although cumulative costs after the first year of treatment were higher in patients who received alteplase than streptokinase, these patients also showed a significantly greater survival benefit. No difference in quality of life was detected by patients on the basis of thrombolytic allocation.
Tolerability
The most significant risk associated with thrombolytic therapy is severe systemic or intracranial haemorrhage. In the GISSI-2/ISG, ISIS-3 and GUSTO trials, the incidence of major bleeding in patients with acute myocardial infarction after treatment with alteplase (or duteplase) was comparable to or less than that seen after streptokinase or anistreplase. In the GISSI-2/ISG and ISIS-3 trials, patients randomised to receive subcutaneous heparin experienced significantly more occurrences of major bleeding, but not stroke, than those who were not.
However, in the GISSI-2/ISG, ISIS-3 and GUSTO trials, total and haemorrhagic stroke occurred more frequently in alteplase (or duteplase) recipients than in those who received streptokinase or anistreplase, especially in patients older than 70 or 75 years. Combination thrombolytic therapy with alteplase and streptokinase increases the risk of stroke compared with monotherapy.
Allergic reactions and hypotension were reported more frequently after administration of streptokinase or anistreplase than alteplase.
Dosage and Administration
Treatment with alteplase should be initiated as soon as possible after the onset of symptoms of acute myocardial infarction. The original dosage regimen for alteplase consists of a 3-hour intravenous infusion where 60mg is administered in the first hour (of which 6 to 10mg is administered as a bolus injection over the first 1 to 2 minutes), 20mg over the second hour and 20mg over the third hour. In the US, smaller patients (< 65kg) should receive a dose of 1.25 mg/kg and, in the UK, patients weighing less than 67kg should receive a total dose of 1.5 mg/kg, both over 3 hours as described above. In addition, the US has now approved the administration of a more rapid (90-minute) infusion of alteplase. The accelerated alteplase dosage schedule in patients > 67kg consists of a 15mg intravenous bolus dose, followed by 50mg infused over the next 30 minutes and then 35mg infused over the next 60 minutes. In lower weight patients (≤ 67kg), the initial alteplase 15mg bolus dose should be followed by a 0.75 mg/kg infusion (not to exceed 50mg) over 30 minutes and then 0.5 mg/kg (not to exceed 35mg) infused over the next 60 minutes.
Similar content being viewed by others
References
Collen D, Lijnen HR, Todd PA, et al. Tissue-type plasminogen activator: a review of its pharmacology and therapeutic use as a thrombolytic agent. Drugs 1989 Sep; 38: 346–88
Granger CB, Califf RM, Topol EJ. Thrombolytic therapy for acute myocardial infarction. A review. Drugs 1992 Sep; 44: 293–325
Hoylaerts M, Rijken DC, Lijnen HR, et al. Kinetics of the activation of plasminogen by human tissue plasminogen activator: role of fibrin. J Biol Chem 1982; 257: 2912–9
Camiolo SM, Thorsen S, Astrup T. Fibrinogenolysis and fibrinolysis with tissue plasminogen activator, urokinase, streptokinase-activated human globulin, and plasmin. Proc Soc Exp Biol Med 1971; 138: 277–80
Miles LA, Plow EF. Binding and activation of plasminogen on the platelet surface. J Biol Chem 1985; 260: 4303–11
Soeda S, Kakiki M, Shimeno H, et al. Some properties of tissue-type plasminogen activator reconstituted onto phospholipid and/or glycolipid vesicles. Biochem Biophys Res Commun 1987; 146: 94–100
Hajjar KA, Hamel NM, Harpel PC, et al. Binding of tissue plasminogen activator to cultured human endothelial cells. J Clin Invest 1987; 80: 1712–9
Tran-Chang C, Wyss P, Kruithof EKO, et al. Tissue-type plasminogen activator increases the binding of plasminogen to clots [abstract]. Haemostasis 1984; 14: 17
Collen D, Lijnen HR. The fibrinolytic system in man. CRC Critical Reviews in Hematology and Oncology 1986; 4: 249–301
Seifried E, Tanswell P, Ellbruck D, et al. Pharmacokinetics and haemostatic status during consecutive infusions of recombinant tissue-type plasminogen activator in patients with acute myocardial infarction. Thromb Haemost 1989 Jun 30; 61: 497–501
Tanswell P, Tebbe U, Neuhaus K-L, et al. Pharmacokinetics and fibrin specificity of alteplase during accelerated infusions in acute myocardial infarction. J Am Coll Cardiol 1992 Apr; 19: 1071–5
Ho CH, Wang SP. Serial thrombolysis-related changes after thrombolytic therapy with tPA in patients with acute myocardial infarction. Thromb Res 1990 May 1; 58: 331–41
Lijnen HR, Marafino Jr BJ, Collen D. In vitro fibrinolytic activity of recombinant tissue-type plasminogen activator in the plasma of various primate species. Thromb Haemost 1984; 52: 308–10
Jan KM, Powers E, Reinhart W, et al. Altered rheological properties of blood following administrations of tissue plasminogen activator and streptokinase in patients with acute myocardial infarction. Adv Exp Med Biol 1990; 281: 409–17
Prisco D, Bonechi F, Scarti L, et al. Thrombolytic versus fibrinogenolytic activity of rt-PA and streptokinase in patients with acute myocardial infarction. Angiology 1990 Aug; 41: 616–20
Collen D, Bounameaux H, De Cock F, et al. Analysis of coagulation and fibrinolysis during intravenous infusion of recombinant human tissue-type plasminogen activator in patients with acute myocardial infarction [abstract no. 38]. Circulation 1986; 73: 57
Matsuo O, Rijken DC, Collen D. Comparison of the relative fibrinogenolytic, fibrinolytic and thrombolytic properties of tissue plasminogen activator and urokinase in vitro. Thromb Haemost 1981; 45: 225–9
Mattsson C, Nyberg-Arrhenius V, Wallen P. Dissolution of thrombi by tissue plasminogen activator, urokinase and streptokinase in an artificial circulating system. Thromb Res 1981; 21: 535–45
Belgian SATG. Effects of alteplase and saruplase on hemostatic variables: a single-blind, randomized trial in patients with acute myocardial infarction. Coron Artery Dis 1991 May; 2: 349–55
Tracy RP, Bovill EG. Fibrinolytic parameters and hemostatic monitoring: identifying and predicting patients at risk for major hemorrhagic events. Am J Cardiol 1992; 69: 52A–9A
Sane DC, Stump DC, Topol EJ, et al. Racial differences in responses to thrombolytic therapy with recombinant tissue-type plasminogen activator. Increased fibrin (ogen)olysis in Blacks. Circulation 1991 Jan; 83: 170–5
Nayak PR, Bhaktaram V, Shetty PK, et al. Third world profile of racial differences in thrombolytic effects of streptokinase [letter]. Circulation 1991; 84: 2205–6
Rijken DC, Hoylaerts M, Collen D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J Biol Chem 1982; 257: 2920–5
Wiman B, Chmielewska J, Ranby M. Inactivation of tissue plasminogen activator in plasma. Demonstration of a complex with a new rapid inhibitor. J Biol Chem 1984; 259: 3644–7
Rapold HJ, Grimaudo V, Declerck PJ, et al. Plasma levels of plasminogen activator inhibitor type 1, beta-thrombo-globulin, and fibrinopeptide A before, during, and after treatment of acute myocardial infarction with alteplase. Blood 1991 Sep 15; 78: 1490–5
Andreotti F, Kluft C, Hackett DR, et al. Thrombin generation after fast or prolonged regimens of tissue-type plasminogen activator [letter]. Lancet 1993 Oct 9; 342: 937–8
Fry ETA, Sobel BE. Lack of interference by heparin with thrombolysis or binding of tissue-type plasminogen activator to thrombi. Blood 1988; 71: 1347–52
Gram J, Munkvad S, Leebeek FW, et al. Reactive coagulation induced by plasmin in patients treated with recombinant tissue-type plasminogen activator. Coron Artery Dis 1993 Apr; 4: 371–7
Dittmar S, Gear ARL. The influence of recombinant tissue-type plasminogen activator and plasmin on platelet aggregation: a quenched-flow study. Fibrinolysis 1993 May; 7: 158–64
Aronson DL, Chang P, Kessler CM. Platelet-dependent thrombin generation after in vitro fibrinolytic treatment. Circulation 1992 May; 85: 1706–12
Prager NA, Torr-Brown SR, Sobel BE, et al. Maintenance of patency after thrombolysis in stenotic coronary arteries requires combined inhibition of thrombin and platelets. J Am Coll Cardiol 1993 Jul; 22: 296–301
Roux SP, Tschopp TB, Kuhn H, et al. Effects of heparin, aspirin and a synthetic platelet glycoprotein IIb–IIIa receptor antagonist (Ro 43-5054) on coronary artery reperfusion and reocclusion after thrombolysis with tissue-type plasminogen activator in the dog. J Pharmacol Exp Ther 1993 Jan; 264: 501–8
Yasuda T, Gold HK, Yaoita H, et al. Comparative effects of aspirin, a synthetic thrombin inhibitor and a monoclonal anti-platelet glycoprotein IIb/IIIa antibody on coronary artery reperfusion, reocclusion and bleeding with recombinant tissue-type plasminogen activator in a canine preparation. J Am Coll Cardiol 1990 Sep; 16: 714–22
Karlberg K-E, Chen J, Hagerman I, et al. Streptokinase, but not tissue plasminogen activator, attenuates platelet aggregation in patients with acute myocardial infarction. J Intern Med 1993 Nov; 234: 513–9
Tanswell P, Seifried E, Stang E, et al. Pharmacokinetics and hepatic catabolism of tissue-type plasminogen activator. Arzneimittel Forschung 1991 Dec; 41: 1310–9
Tanswell P, Seifried E, Su PC, et al. Pharmacokinetics and systemic effects of tissue-type plasminogen activator in normal subjects. Clin Pharmacol Ther 1989 Aug; 46: 155–62
Kerins DM, Roy L, Kunitada S, et al. Pharmacokinetics of tissue-type plasminogen activator during acute myocardial infarction in men. Effect of a prostacyclin analogue. Circulation 1992 Feb; 85: 526–32
Genentech I. Alteplase recombinant prescribing information. South San Fransisco, California, USA, 1995.
Pasternak RC, Braunwald E. Acute Myocardial Infarction. In: Wilson JD, Braunwald E, Isselbacher KJ, et al., editors. Harrison’s principles of internal medicine. 12th ed. v. 1. New York: McGraw-Hill, Inc., 1991: 953–64
Gonzalez ER, Sypniewski E. Acute myocardial infarction: diagnosis and treatment. In: DiPiro JT, Talbert RL, Hayes PE, et al., editors. Pharmacotherapy: a pathophysiologic approach. New York: Elsevier Science Publishing, 1989: 213–33
The International Study Group. In-hospital mortality and clinical course of 20 891 patients with suspected acute myocardial infarction randomised between alteplase and streptokinase with or without heparin. Lancet 1990 Jul 14; 336: 71–5
GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993 Sep 2; 329: 673–82
Third International Study of Infarct Survival Collaborative Group. ISIS-3: a randomised comparison of streptokinase vs tissue plasminogen activator vs anistreplase and of aspirin plus heparin vs aspirin alone among 41 299 cases of suspected acute myocardial infarction. Lancet 1992 Mar 28; 339: 753–70
Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity from all the randomised trials of more than 1000 patients. Lancet 1994 February 5; 343: 311–22
Braunwald E. The open-artery theory is alive and well — again. N Engl J Med 1993 November 25; 329: 1650–2
Grünewald M, Seifried E. Meta-analysis of all available published clinical trials (1958–1990) on thrombolytic therapy in AMI: relative efficacy of different therapeutic strategies. Fibrinolysis 1994; 8: 67–86
Topol EJ, Agnelli G. Strategies for administration of tissue plasminogen activator. Mol Biol Med 1991 Apr; 8: 219–34
Anderson JL. Overview of patency as an end point of thrombolytic therapy. Am J Cardiol 1991 December 5; 67: 11E–6E
GUSTO Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med 1993 Nov 25; 329: 1615–22
Cannon CP, McCabe CH, Diver DJ, et al. Comparison of front-loaded recombinant tissue-type plasminogen activator, anistreplase and combination thrombolytic therapy for acute myocardial infarction: results of the Thrombolysis in Myocardial Infarction (TIMI) 4 trial. J Am Coll Cardiol 1994 December; 24: 1602–10
Carney RJ, Murphy GA, Brandt TR, et al. Randomized angiographic trial of recombinant tissue-type plasminogen activator (alteplase) in myocardial infarction. J Am Coll Cardiol 1992 Jul; 20: 17–23
Neuhaus K-L, von Essen R, Tebbe U, et al. Improved thrombolysis in acute myocardial infarction with front-loaded administration of alteplase: results of the rt-PA-APSAC Patency Study (TAPS). J Am Coll Cardiol 1992 Apr; 19: 885–91
Wall TC, Califf RM, George BS. Accelerated plasminogen activator dose regimens for coronary thrombolysis. J Am Coll Cardiol 1992 Mar 1; 19: 482–9
Grines CL, Nissen SE, Booth DC, et al. A prospective, randomized trial comparing combination half-dose tissue-type plasminogen activator and streptokinase with full-dose tissue-type plasminogen activator. Circulation 1991 Aug; 84: 540–9
Bode C, Baumann H, von Hodenberg E. Combinations of thrombolytic agents in acute myocardial infarction. Z Kardiol 1993; 82 Suppl. 2: 125–8
Califf RM, Topol EJ, Stack RS, et al. Evaluation of combination thrombolytic therapy and timing of cardiac catheterization in acute myocardial infarction. Results of thrombolysis and angioplasty in myocardial infarction — phase 5 randomized trial. Circulation 1991 May; 83: 1543–56
The Urokinase and Alteplase in Myocardial Infarction Collaborative Group. Combination of urokinase and alteplase in the treatment of myocardial infarction. Coron Artery Dis 1991; 2: 225–35
Van de Werf F. Discrepancies between the effects of coronary reperfusion on survival and left ventricular function. Lancet 1989 June 17: 1367–9
Verstraete M. Unresolved issues in the thrombolytic treatment of myocardial infarction. Acta Cardiol 1991 Oct; 46: 517–26
Mortelmans L, Vanhaecke J, Lesaffre E, et al. Evaluation of the effect of thrombolytic treatment on infarct size and left ventricular function by enzymatic, scintigraphic, and angiographic methods. Am Heart J 1990 Jun; 119: 1231–7
Picard MH, Wilkins GT, Ray P, et al. Long-term effects of acute thrombolytic therapy on ventricular size and function. Am Heart J 1993 Jul; 126: 1–10
Otto CM, Stratton JR, Maynard C, et al. Echocardiographic evaluation of segmental wall motion early and late after thrombolytic therapy in acute myocardial infarction: the Western Washington Tissue Plasminogen Activator Emergency Room trial. Am J Cardiol 1990 Jan 15; 65: 132–8
Topol EJ, Califf RM, Vandormael M. A randomized trial of late reperfusion therapy for acute myocardial infarction. Circulation 1992 Jun; 85: 2090–9
Villari B, Piscione F, Bonaduce D, et al. Usefulness of late coronary thrombolysis (recombinant tissue-type plasminogen activator) in preserving left ventricular function in acute myocardial infarction. Am J Cardiol 1990 Dec 1; 66: 1281–6
Cross DB, Ashton NG, Norris RM, et al. Comparison of the effects of streptokinase and tissue plasminogen activator on regional wall motion after first myocardial infarction: analysis by the centerline method with correction for area at risk. J Am Coll Cardiol 1991 Apr; 17: 1039–46
Gruppo Italiano per Studio della Sopravvivenza nell’Infarto Miocardico. GISSI-2: a factorial randomised trial of alteplase versus streptokinase and heparin versus no heparin among 12,490 patients with acute myocardial infarction. Lancet 1990 Jul 14; 336: 65–71
Bassand J-P, Cassagnes J, Machecourt J, et al. Comparative effects of APSAC and rt-PA on infarct size and left ventricular function in acute myocardial infarction. A multicenter randomized study. Circulation 1991 Sep; 84: 1107–17
Anderson JL, Becker LC, Sorensen SG. Anistreplase versus alteplase in acute myocardial infarction: comparative effects on left ventricular function, morbidity and 1-day coronary artery patency. J Am Coll Cardiol 1992 Oct; 20: 753–66
Grines CL, Browne KF, Marco J. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. N Engl J Med 1993 Mar 11; 328: 673–9
Arnold AER, Serruys PW, Rutsch W, et al. Reasons for the lack of benefit of immediate angioplasty during recombinant tissue plasminogen activator therapy for acute myocardial infarction: a regional wall motion analysis. J Am Coll Cardiol 1991 Jan; 17: 11–21
Barbash GI, Roth A, Hod H, et al. Randomized controlled trial of late in-hospital angiography and angioplasty versus conservative management after treatment with recombinant tissue-type plasminogen activator in acute myocardial infarction. Am J Cardiol 1990 Sep 1; 66: 538–45
White HD, Norris RM, Brown MA, et al. Left ventricular endsystolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 1987; 76(1): 44–51
Morgan CD, Roberts RS, Haq A, et al. Coronary patency, infarct size and left ventricular function after thrombolytic therapy for acute myocardial infarction: results from the Tissue Plasminogen Activator: Toronto (TPAT) placebo-controlled trial. J Am Coll Cardiol 1991 Jun; 17: 1451–7
Marino P, Destro G, Barbieri E, et al. Reperfusion of the infarctrelated coronary artery limits left ventricular expansion beyond myocardial salvage. Am Heart J 1992 May; 123: 1157–65
Henzlova MJ, Bourge RC, Papapietro SE, et al. Long-term effect of thrombolytic therapy on left ventricular ejection fraction after acute myocardial infarction. Am J Cardiol 1991 Jun 15; 67: 1354–9
Zaret BL, Wackers FJT, Terrin ML, et al. Assessment of global and regional left ventricular performance at rest and during exercise after thrombolytic therapy for acute myocardial infarction: results of the thrombolysis in myocardial infarction (TIMI) II study. Am J Cardiol 1992 Jan 1; 69: 1–9
Arnold AE, Simoons ML, Van-de-Werf F, et al. Recombinant tissue-type plasminogen activator and immediate angioplasty in acute myocardial infarction. One-year follow-up. The European Cooperative Study Group [published errata appear in Circulation 1993 May; 87 (5): 1775 and 1993 Jun; 87 (6): 2070]. Circulation 1992 Jul; 86: 111–20
Califf RM, Topol EJ, George BS, et al. One-year outcome after therapy with tissue plasminogen activator: report from the Thrombolysis and Angioplasty in Myocardial Infarction trial. Am Heart J 1990 Apr; 119: 777–85
GISSI-2 and International Study Group. Six-month survival in 20,891 patients with acute myocardial infarction randomized between alteplase and streptokinase with or without heparin. Eur Heart J 1992 Dec; 13: 1692–7
Herlitz J, Dellborg M, Hartford M, et al. Mortality and morbidity 1 year after early thrombolysis in suspected AMI: results from the TEAHAT Study. J Intern Med Suppl 1991; 734: 43–51
Müller DWM, Topol EJ, George BS, et al. Two-year outcome after angiographically documented myocardial reperfusion for acute coronary occlusion. Am J Cardiol 1990 Oct 1; 66: 796–801
Rogers WJ, Bairn DS, Gore JM, et al. Comparison of immediate invasive, delayed invasive, and conservative strategies after tissue-type plasminogen activator. Results of the Thrombolysis in Myocardial Infarction (TIMI) phase II-A trial. Circulation 1990 May; 81: 1457–76
Wilcox RG, von der Lippe G, Olsson CG, et al. Effects of alteplase in acute myocardial infarction: 6-month results from the ASSET study. Anglo-Scandinavian Study of Early Thrombolysis [see comments]. Lancet 1990 May 19; 335: 1175–8
Williams DO, Braunwald E, Knatterud G, et al. One-year results of the Thrombolysis in Myocardial Infarction Investigation (TIMI) Phase II Trial. Circulation 1992 Feb; 85: 533–42
Terrin ML, Williams DO, Kleiman NS, et al. Two- and three-year results of the Thrombolysis in Myocardial Infarction (TIMI) phase II clinical trial. J Am Coll Cardiol 1993 Dec; 22: 1763–72
GUSTO: 1-year results. Sydney: Adis International, 1994 (Lysis Update; Issue 8)
Muller DWM, Topol EJ. Thrombolytic therapy: adjuvant mechanical intervention for acute myocardial infarction. Am J Cardiol 1992 January 3; 69: 60A–70A
Ross AM. The current controversies regarding reperfusion therapy for acute myocardial infarction. Z Kardiol 1993; 82 Suppl. 2: 113–7
Little T, Lee K, Mukherjee D, et al. Delayed coronary angioplasty after thrombolytic therapy for acute myocardial infarction. Am J Cardiol 1990 Nov 15; 66: 1259–60
van den Brand MJ, Betriu A, Bescos LL, et al. Randomized trial of deferred angioplasty after thrombolysis for acute myocardial infarction. Coron Artery Dis 1992 May; 3: 393–401
Vacek JL, Rosamond TL, Kramer PH, et al. Direct angioplasty versus initial thrombolytic therapy for acute myocardial infarction: long-term follow-up and changes in practice pattern. Am Heart J 1992 Dec; 124: 1411–8
Ohman EM, Califf RM, Topol EJ, et al. Consequences of reocclusion after successful reperfusion therapy in acute myocardial infarction. Circulation 1990 Sep; 82: 781–91
Grambow DW, Topol EJ. Selecting patients for thromblytic therapy in acute MI. Drug Ther 1992 January; 22: 37–46
American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee to Develop Guidelines for the Early Management of Patients with Acute Myocardial Infarction). ACC/AHA guidelines for the early management of patients with acute myocardial infarction. Circulation 1990; 82(2): 664–707
Becker RC, Corrao JM, Harrington R, et al. Recombinant tissue-type plasminogen activator: current concepts and guidelines for clinical use in acute myocardial infarction. Part I. Am Heart J 1991 Jan; 121: 220–44
Taylor GJ, Moses HW, Koester D, et al. A difference between front-loaded streptokinase and standard-dose recombinant tissue-type plasminogen activator in preserving left ventricular function after acute myocardial infarction (the Central Illinois Thrombolytic Therapy study). Am J Cardiol 1993 Nov 1; 72: 1010–4
Hathaway WR, Zabel KM, Peterson ED, et al. Incremental prognostic value of electrocardiographic findings when added to baseline clinical variables in patients with acute myocardial infarction [abstract no. 908-109]. J Am Coll Cardiol 1995 February; 25
ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17 187 cases of suspected acute myocardial infarction. Lancet 1988 August 13: 349-60
De Bono DP, Hopkins A, for the Joint Audit Committee of the British Cardiac Society and the Royal College of Physicians. The management of acute myocardial infarction: guidelines and audit standards. J R Coll Physicians Lond 1994 July/August; 28(4): 312–7
Prins MH, Hirsh J. Heparin as an adjunctive treatment after thrombolytic therapy for acute myocardial infarction. Am J Cardiol 1991 Jan 25; 67: 3A–11A
Hirsch J. Heparin. N Engl J Med 1991 May 30; 324: 1565–74
Sobel BE, Hirsh J. Principles and practice of coronary thrombolysis and conjunctive treatment. Am J Cardiol 1991 Aug 1; 68: 382–8
Sobel BE, Collen D. Strokes, statistics and sophistry in trials of thrombolysis for acute myocardial infarction. Am J Cardiol 1993 Feb 15; 71: 424–7
Bleich SD, Nichols TC, Schumacher R, et al. Effect of heparin on coronary arterial patency after thrombolysis with tissue plasminogen activator in acute myocardial infarction. Am J Cardiol 1990 Dec 15; 66: 1412–7
Hsia J, Hamilton WP, Kleiman N, et al. A comparison between heparin and low-dose aspirin as adjunctive therapy with tissue plasminogen activator for acute myocardial infarction. N Engl J Med 1990 Nov 22; 323: 1433–7
Henahan S. The GUSTO results. The pendulum swings. New Ethicals 1993 Aug; 30: 89–92
The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) IIa Investigators. Randomized trial of intravenous heparin versus recombinant hirudin for acute coronary syndromes. Circulation 1994; 90: 1631–7
Antman EM, TIMI 9A Investigators. Hirudin in acute myocardial infarction: safety report from the thrombolysis and thrombin inhibition in myocardial infarction (TIMI) 9A trial. Circulation 1994; 90: 1624–30
Neuhaus KL, v. Essen R, Tebbe U, et al. Safety observations from the pilot phase of the randomized r-hirudin for improvement of thrombolysis (HIT-III) study. A study of the Arbeitsgemeinschaft Leitender Kardiologischer Krankenhausärzte (ALKK). Circulation 1994; 90: 1638–42
Canadian Consensus Conference on Coronary Thrombolysis: Recommendations. Can J Cardiol 1993 July/August; 9: 559-65
Kennedy JW, Weaver WD. Potential use of thrombolytic therapy before hospitalization. Am J Cardiol 1989 Jul 5; 64: 8A-11A (discussion 24A–26A)
Risenfors M, Hartford M, Dellborg M, et al. Effect of early intravenous rt-PA on infarct size estimated from serum enzyme activity: results from the TEAHAT Study. J Intern Med 1991; 229 Suppl. 734: 11–8
The Thrombolysis Early in Acute Heart Attack Trial Study Group. Very early thrombolytic therapy in suspected acute myocardial infarction. Am J Cardiol 1990 Feb 15; 65: 401–7
Weaver WD, Cerqueira M, Hallstrom AP. Prehospital-initiated vs hospital-initiated thrombolytic therapy. The Myocardial Infarction Triage and Intervention trial. JAMA 1993 Sep 8; 270: 1211–6
McNeill AJ, Flannery DJ, Wilson CM, et al. Thrombolytic therapy within one hour of the onset of acute myocardial infarction. Q J Med 1991 Jun; 79: 487–94
Barbash GI, Roth A, Hod H, et al. Improved survival but not left ventricular function with early and prehospital treatment with tissue plasminogen activator in acute myocardial infarction. Am J Cardiol 1990 Aug 1; 66: 261–6
Topol EJ, Califf RM. More on the GUSTO trial [letter] [published erratum appears in NEJM 1994; 331 (10): 687]. N Engl J Med 1994; 331(4): 277–8
Risenfors M, Gustavsson G, Ekstrom L, et al. Prehospital thrombolysis in suspected acute myocardial infarction: results from the TEAHAT Study. J Intern Med 1991; 229 Suppl. 734: 3–10
Bouten MJM, Simoons ML, Hartman JAM, et al. Prehospital thrombolysis with alteplase (rt-PA) in acute myocardial infarction. Eur Heart J 1992 Jul; 13: 925–31
Gore JM. Patient selection issues in the use of thrombolytic therapy in patients with acute myocardial infarction. Z Kardiol 1993; 82 Suppl. 2: 143–6
Bonaduce D, Petretta M, Bianchi V, et al. Left ventricular remodeling and function after myocardial infarction. Prim Cardiol 1993; 19(6): 41–8
Bonaduce D, Petretta M, Villari B, et al. Effects of late administration of tissue-type plasminogen activator on left ventricular remodeling and function after myocardial infarction. J Am Coll Cardiol 1990 Dec; 16: 1561–8
Hampton J, Wilcox R, Armstrong P. Late Assessment of Thrombolytic Efficacy (LATE) study with alteplase 6-24 hours after onset of acute myocardial infarction. Lancet 1993 Sep 25; 342: 759–66
Williamson BD, Muller DW, Topol EJ. Should older patients with acute myocardial infarction receive thrombolytic therapy. Drugs Aging 1992; 2(6): 461–8
Aguirre FV, McMahon RP, Mueller H, et al. Impact of age on clinical outcome and postlytic management strategies in patients treated with intravenous thrombolytic therapy. Results from the TIMI II study. Circulation 1994 July; 90: 78–86
White HD. Acute myocardial infarction in the elderly: weighing the risks and opportunities. Proceedings, Myocardial Reperfusion Concepts and Controversies 8th Annual International Symposium, The Cleveland Clinic Foundation, Cleveland, Ohio 1995: 59–60
Maggioni AP, Maseri A, Fresco C, et al. Age-related increase in mortality among patients with first myocardial infarctions treated with thrombolysis. N Engl J Med 1993 Nov 11; 329: 1442–8
Weaver WD, Litwin PE, Martin JS, et al. Effect of age on use of thrombolytic therapy and mortality in acute myocardial infarction. J Am Coll Cardiol 1991; 18: 657–62
Cragg DR, Friedman HZ, Bonema JD, et al. Outcome of patients with acute myocardial infarction who are ineligible for thrombolytic therapy. Ann Intern Med 1991 August 1; 115: 173–7
Woo KS, White HD. Pharmacoeconomic aspects of treatment of acute myocardial infarction with thrombolytic agents. PharmacoEconomics 1993 Mar; 3: 192–204
Levin L-Å, Jönsson B. Cost-effectiveness of thrombolysis — a randomized study of intravenous rt-PA in suspected myocardial infarction. Eur Heart J 1992 Jan; 13: 2–8
Machecourt J, Dumoulin J, Calop J, et al. Cost effectiveness of thrombolytic treatment for myocardial infarction: comparison of anistreplase, alteplase and streptokinase in 270 patients treated within 4 hours. Eur Heart J 1993 Jan; 14: 75–83
Mark DB, Hlatky MA, Califf RM, et al. Cost effectiveness of thrombolytic therapy with tissue plasminogen activator as compared with streptokinase for acute myocardial infarction. N Engl J Med 1995 May 25; 332: 1418–24
Califf RM, Fortin DF, Tenaglia AN, et al. Clinical risks of thrombolytic therapy. Am J Cardiol 1992 January 3; 69: 12A–20A
de Bono DP. Complications of thrombolysis and their clinical management. Z Kardiol 1993; 82 Suppl. 2: 147–51
Morris JA, Muller DWM, Topol EJ. Combination thrombolytic therapy: a comparison of simultaneous and sequential regimens of tissue plasminogen activator and urokinase. Am Heart J 1991 Aug; 122: 375–80
Simoons ML, De JP, Van DR, et al. Intracranial hemorrhage after thrombolytic therapy. A perspective. Z Kardiol 1993; 82 Suppl. 2: 153–6
Gore JM, Sloan M, Price TR, et al. Intracerebral hemorrhage, cerebral infarction, and subdural hematoma after acute myocardial infarction and thrombolytic therapy in the thrombolysis in myocardial infarction study. Thrombolysis in myocardial infarction, phase II, pilot and clinical trial. Circulation 1991 Feb; 83: 448–59
Nicolini FA, Ferrini D, Ottani F, et al. Concurrent nitroglycerin therapy impairs tissue-type plasminogen activator-induced thrombolysis in patients with acute myocardial infarction. Am J Cardiol 1994 October 1; 74: 662–6
Gupta BK, Spinowitz BS, Charytan C, et al. Cholesterol crystal embolization-associated renal failure after therapy with recombinant tissue-type plasminogen activator. Am J Kidney Dis 1993 Jun; 21: 659–62
Arora RR, Magun AM, Grossman M. Cholesterol embolization syndrome after intravenous tissue plasminogen activator for acute myocardial infarction. Am Heart J 1993 Jul; 126: 225–8
Diethelm A, Vorburger C, Anabitarte M, et al. Cholesterol crystal embolization as a complication of fibrinolytic treatment of acute myocardial infarction. Schweiz Med Wochenschr 1994 Aug 20; 124: 1437–41
Ben-Chitrit S, Korzets Z, Hershkovitz R, et al. Cholesterol embolization syndrome following thrombolytic therapy with streptokinase and tissue plasminogen activator. Nephrol Dial Transplant 1994; 9(4): 428–30
Blankenship JC, Butler M, Garbes A. Prospective assessment of cholesterol embolization in patients with acute myocardial infarction treated with thrombolytic vs conservative therapy. Chest 1995 March; 107: 662–8
Cardiovascular system. In: British Medical Association, Royal Pharmaceutical Society of Great Britain, editors. British National Formulary, v. 29. London: The Pharmaceutical Press, 1995: 105
McKendall GR, Attubato MJ, Drew TM, et al. Safety and efficacy of a new regimen of intravenous recombinant tissue-type plasminogen activator potentially suitable for either prehospital or in-hospital administration. J Am Coll Cardiol 1991 Dec; 18: 1774–8
Purvis JA, Trouton TG, Roberts MJD, et al. Effectiveness of double bolus alteplase in the treatment of acute myocardial infarction. Am J Cardiol 1991 Dec 15; 68: 1570–4
Tranchesi B, Verstraete M, Vanhove P, et al. Intravenous bolus administration of recombinant tissue plasminogen activator to patients with acute myocardial infarction. Coron Artery Dis 1990 Jan–Feb; 1: 83–8
Gemmill JD, Hogg KJ, MacIntyre PD, et al. A pilot study of the efficacy and safety of bolus administration of alteplase in acute myocardial infarction. Br Heart J 1991 Aug; 66: 134–8
Smalling RW, Schumacher R, Morris D, et al. Improved infarct-related arterial patency after high dose, weight-adjusted, rapid infusion of tissue-type plasminogen activator in myocardial infarction: results of a multicenter randomized trial of two dosage regimens. J Am Coll Cardiol 1990 Apr; 15: 915–21
Gibelin P, Tiger F, Moles V, et al. Influence of the rt-PA dose (1 mg/kg versus 1.5 mg/kg) and duration of administration on the patency of infarct-related coronary arteries in 81 patients. Cardiovasc Drugs Ther 1992 Aug; 6: 373–7
Hennekens CH. Thrombolytic therapy: pre- and post-GISSI-2, ISIS-3, and GUSTO-1. Clin Cardiol 1994 Jan; 17 Suppl. I: 115–17
Benson NH, Maningas PA, Krohmer JR, et al. Guidelines for the prehospital use of thrombolytic agents. Ann Emerg Med 1994; 23: 1047–8
Battershill PE, Benfield P, Goa KL. Streptokinase. A review of its pharmacology and therapeutic efficacy in acute myocardial infarction in older patients. Drugs Aging 1994 Jan; 4(1): 63–86
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: F. Andreotti, Facoltà di Medicina e Chirurgia ‘Agostino Gemelli’, Università Cattolica del Sacro Cuore, Rome, Italy; D.P. de Bono, Department of Cardiology, Glenfield General Hospital, Leicester, England; S.R.M. Holmberg, Cardiac Department, Royal Sussex City Hospital, Brighton, Sussex, England; P. Sleight, Cardiac Department, John Radcliffe Hospital, Oxford, England; B.E. Sobel, Department of Medicine, Medical Center Hospital of Vermont, Burlington, Vermont, USA; A.J. Tiefenbrunn, Cardiovascular Division, Washington University School of Medicine/Barnes Hospital, St. Louis, Missouri, USA; E.J. Topol, Department of Cardiology, The Cleveland Clinic Foundation, Cleveland, Ohio, USA; H.D. White, Cardiology Department, Green Lane Hospital, Auckland, New Zealand; R.G. Wilcox, Division of Cardiovascular Medicine, University Hospital, Nottingham, England.
Rights and permissions
About this article
Cite this article
Gillis, J.C., Wagstaff, A.J. & Goa, K.L. Alteplase. Drugs 50, 102–136 (1995). https://doi.org/10.2165/00003495-199550010-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199550010-00008