Abstract
The role of postprandial hyperglycemia (PPHG) in diabetes mellitus is being increasingly recognized. It is known that PPHG contributes to the increased risk of both micro- and macrovascular complications in patients with diabetes mellitus. This review looks at the clinical significance of PPHG and the currently available therapeutic modalities.
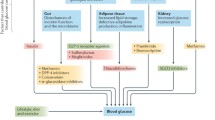
The causes of PPHG are influenced by many factors which include a rapid flux of glucose from the gut, impaired insulin release, endogenous glucose production by the liver and peripheral insulin resistance. Knowledge of the pathophysiology of PPHG is essential when adopting treatment options to tackle the problem. Although most oral antihyperglycemic agents and insulins lower both fasting and postprandial blood glucose levels, drugs are now available which specifically act to control PPHG. These drugs may be classified based on the site of their action. α-Glucosidase inhibitors like acarbose and miglitol attenuate the rate of absorption of sucrose by acting on the luminal enzymes. Adverse effects of these agents are predominantly gastrointestinal. Newer insulin secretagogues have been developed which attempt to mimic the physiological release of insulin and thus ameliorate PPHG. These include third generation sulfonylureas like glimepiride and nonsulfonylurea secretagogues like repaglinide and nateglinide. Rapid-acting insulin analogs, the amino acid sequences of which have been altered such that they have a faster onset of action, help to specifically target PPHG. Pre-mixed formulations of the analogs have also been developed.
Finally, drugs under development which hold promise in the management of patients with PPHG include pramlintide, an amylin analog, and glucagon-like peptide-1 and its analogs.







Similar content being viewed by others
References
Jones DB, Gill GV. Non insulin dependent diabetes mellitus. In: Pickup J, Williams G, editors. Text book of diabetes mellitus. 2nd ed. London: Blackwell Sciences Ltd, 1997: 17.6–13
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin dependent diabetes mellitus. N Engl J Med 1993; 329: 977–86
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–53
Shichiri M, Kishikawa H, Ohkubo Y, et al. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000; 23Suppl. 2: B21–9
Slama G. Clinical significance of post-prandial blood glucose excursions in Type 1 and Type 2 diabetes mellitus. Int J Clin. Pract Suppl 2000; 112: 9–12
Patel V, Rassam SM, Chen HC, et al. Oxygen reactivity in diabetes mellitus: effect of hypertension and hyperglycemia. Clin Sci (Lond) 1994; 86: 689–95
Sullivan PM, Davies GE, Caldwell G, et al. Retinal blood flow during hyperglycemia: a laser doppler velocimetry study. Invest Ophthalmol Vis Sci 1990; 31: 2041–5
Tuttle KR, Bruton JL, Perusek MC, et al.. Effect of strict glycemic control on renal hemodynamic response to amino acids and renal enlargement in insulin dependent diabetes mellitus. N Engl J Med 1991; 324: 1626–32
Orskov L, Worm M, Schmitz O, et al. Nerve conduction velocity in man: influence of glucose, somatostatin and electrolytes. Diabetologia 1994; 37: 1216–20
Yeap BB, Russo A, Fraser RJ, et al. Hyperglycemia affects cardiovascular autonomic nerve function in normal subjects. Diabetes Care 1996; 19: 880–2
Pettitt DJ, Knowler WC, Lisse JR, et al. Development of retinopathy and proteinuria in relation to plasma glucose concentrations in Pima Indians. Lancet 1980; II: 1050–2
Jarrett RJ, Keen H. Hyperglycemia and diabetes mellitus. Lancet 1976; II: 1009–12
Teuscher A, Schnell H, Wilson PW. Incidence of diabetic retinopathy and relationship to baseline plasma glucose and blood pressure. Diabetes Care 1988; 11: 246–51
Hanefeld M, Temelkova-Kurktschiev T, Schaper F, et al. Impaired fasting glucose is not a risk factor for atherosclerosis. Diabet Med 1999; 16: 212–8
Donahue RP, Abbott RD, Reed DM, et al. Post challenge glucose concentration and coronary heart disease in men of Japanese ancestry: Honolulu Heart Program. Diabetes 1987; 36: 689–92
Fuller JH, Shipley MJ, Rose G, et al. Coronary heart-disease risk and impaired glucose tolerance: the Whitehall Study. Lancet 1980; I: 1373–6
Jackson CA, Yudkin JS, Forrest RD. A comparison of the relationships of the glucose tolerance test and the glycated hemoglobin assay with diabetic vascular disease in the community: the Islington Diabetes Survey. Diabetes Res Clin Pract 1992; 17(2): 111–23
Jarrett RJ, McCartney P, Keen H. The Bedford Survey: ten year mortality rates in newly diagnosed diabetics, borderline diabetics and normoglycemic controls and risk indices for coronary heart disease in borderline diabetics. Diabetologia 1982; 22: 79–84
Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11 year follow-up. Diabetologia 1996 39: 1577–83
de Vegt F, Dekker JM, Ruhe HG, et al. Hyperglycemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia 1999; 42: 926–31
DECODE study group, on behalf of the European Diabetes Epidemiology Group. Diabetes Epidemiology: collaborative analysis of Diagnostic Criteria in Europe. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association Diagnostic criteria. Lancet. 1999; 354: 617–21
Fontbonne AM, Eschwege EM. Insulin and cardiovascular disease: Paris Prospective Study. Diabetes Care 1991; 14: 461–9
Pyorala K, Savolainen E, Kaukola S, et al. Plasma insulin as coronary heart disease risk factor: relationship to other risk factors and predictive value during 9½ year follow-up of the Helsinki Policemen Study population. Acta Med Scand Suppl 1985; 701: 38–52
Groot PH, van Stiphout WA, Krauss XH, et al. Postprandial lipoprotein metabolism in normolipidemic men with and without coronary artery disease. Arterioscler Thromb 1991; 11: 653–62
Patsch JR, Miesenbock G, Hopferweiser T, et al. Relation of triglyceride metabolism and coronary artery disease: studies in the postprandial state. Arterioscler Thromb 1992; 12: 1336–45
Ginsberg HN, Jones J, Blaner WS, et al. Association of postprandial triglyceride and retinyl palmitate responses with newly diagnosed exercise: induced myocardial ischaemia in middle aged men and women. Arterioscler Thromb Vasc Biol 1995; 15: 1829–38
Habib MP, Dickerson FD, Mooradian AD. Effect of diabetes, insulin and glucose load on lipid peroxidation in the rat. Metabolism 1994; 43: 1442–5
Wehmeier KR, Mooradian AD. Autooxidative and antioxidative potential of simple carbohydrates. Free Radie Biol Med 1994; 17: 83–6
Mooradian AD, Habib MP, Dickerson F. Effect of simple carbohydrates, casein in hydrolysate and a lipid test meal on ethane exhalation rate. J Appl Physiol 1994; 76: 1119–22
Mooradian AD, Thurman JE. Drug therapy of postprandial hyperglycemia d. Drugs 1999; 57: 19–29
Del Prato S, Enzi G, Vigili de Kreutzenberg S, et al. Insulin regulation of glucose and lipid metabolism in massive obesity. Diabetologia 1990; 33: 228–36
Lillioja S, Mott DM, Howard BV, et al. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med 1988; 318: 1217–25
Eriksson J, Franssila-Kallunki A, Ekstrand A, et al. Early metabolic defects in persons at increased risk for non insulin dependent diabetes mellitus. N Engl J Med 1989; 321: 337–43
Gulli G, Ferrannini E, Stern M, et al. The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican American NIDDM parents. Diabetes 1992; 41: 1575–86
Cerasi E, Luft R. The plasma insulin response to glucose infusion in healthy subjects and in diabetes mellitus. Acta Endocrinol (Copenh) 1967; 55: 278–304
Davies MJ, Rayman G, Grenfell A, et al. Loss of first phase insulin response to intravenous glucose in subjects with persistent impaired glucose tolerance. Diabet Med 1994; 11: 432–6
Kishimoto M, Yamasaki Y, Kubota M, et al. 1,5-Anhydro-D-glucitol evaluates daily glycemie excursions in well-controlled NIDDM. Diabetes Care 1995; 18: 1156–9
American Diabetes Association. Nutrition Recommendations and principles for people with diabetes mellitus. Diabetes Care 2000; 23Suppl. 1: S43–6
Balfour JA, McTavish D. Acarbose: an update of its pharmacology and therapeutic use in diabetes mellitus. Drugs 1993; 46: 1025–54
Clissold SP, Edwards C. Acarbose: a preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs 1988; 35(3): 214–43z
Hayakawa T, Kondo T, Okumura N, et al. Enteroglucagon release in disaccharide malabsorption induced by intestinal alpha-glucosidase inhibition. Am J Gastroenterol 1989; 84: 523–6
Jenkins DJ, Taylor RH, Goff DV, et al. Scope and specificity of acarbose in slowing carbohydrate absorption in man. Diabetes 1981; 30: 951–4
Couet C, Ulmer M, Hamdaoui M, et al. Metabolic effects of acarbose in young healthy men. Eur J Clin Nutr 1989; 43: 187–96
Hanefeld M, Fischer S, Schulze J, et al. Therapeutic potentials of acarbose as first line drug in NIDDM insufficiently treated with diet alone. Diabetes Care 1991; 14: 732–7
Hotta N, Kakuta H, Sano T, et al. Long-term effect of acarbose on glycemic control in non-insulin-dependent diabetes mellitus: a placebo-controlled double blind study. Diabet Med 1993; 10: 134–8
Nestel PJ, Bazelmans J, Reardon MF, et al. Lower triglyceride production during carbohydrate-rich diets through acarbose, a glucoside hydrolase inhibitor. Diabete Metab 1985; 11: 316–7
Walter-Sack IE, Wolfram G, Zollner N. Effects of acarbose on serum lipoproteins in healthy individuals during prolonged administration of a fiber-free formula diet. Ann Nutr Metab 1989; 33: 100–7
Gerard J, Lefebvre PJ, Luyckx AS. Glibenclamide pharmacokinetics in acarbose-treated type 2 diabetics.Eur J Clin Pharmacol 1984; 27: 233–6
Scheen AJ, de Magalhaes AC, Salvatore T, et al. Reduction of acute bioavailability of metformin by the alpha-glucosidase inhibitor acarbose in normal man. Eur J Clin Invest 1994; 24Suppl. 3: 50–4
Scott LJ, Spencer CM. Miglitol: a review of its therapeutic potential in type 2 diabetes mellitus. Drugs 2000; 59: 521–49
Johnston PS, Coniff RF, Hoogwerf BJ, et al. Effects of the carbohydrase inhibitor miglitol in sulfonylurea-treated NIDDM patients. Diabetes Care 1994; 17: 20–9
Pagano G, Marena S, Corgiat-Mansin L, et al. Comparison of miglitol and glibenclamide in the diet-treated type 2 diabetic patients. Diabetes Metab 1995; 21: 162–7
Segal P, Feig PU, Schernthaner G, et al. The efficacy and safety of miglitol therapy compared with glibenclamide in patients with NIDDM inadequately controlled by diet alone. Diabetes Care 1997; 20: 687–91
Van Gaal L, Maislos M, Schernthaner G, et al. Miglitol combined with metformin improves glycemic control in Type 2 diabetes. Diabetes Obes Metab 2001; 3: 326–31
Rybka J, Goke B, Sissmann J. European comparative study of 2α-glucosidase inhibitors, miglitol and acarbose [abstract]. Daibetes 1999; 48Suppl. 1: 101
Salvatore T, Scheen AJ, Ferreira Alves de Magalhaes AC, et al. Slight modification of the pharmacokinetic parameters of glibenclamide after treatment with the alpha glucosidase inhibitor miglitol in normal subjects. Therapie 1990; 45: 365
Standl E, Schernthaner G, Rybka J, et al. Improved glycaemic control with miglitol in type 2 diabetics insufficiently controlled on oral hypoglycemics. Diabetologia 1999; 1: 223
Gregorio F, Ambrosi F, Cristallini S, et al. Therapeutic concentrations of tolbutamide, glibenclamide, gliclazide and gliquidone at different glucose levels: in vitro effects on pancreatic A and B cell function. Diabetes Res Clin Pract 1992; 18: 197–206
Muller G, Hartz D, Punter J, et al. Differential interaction of glimepiride and glibenclamide with the beta cell sulfonylurea receptor: I. Binding characteristics. Biochim Biophys Acta 1994; 1191: 267–77
Kilo C, Dudley J, Kalb B. Evaluation of the efficacy and safety of Diamicron in non-insulin-dependent diabetic patients. Diabetes Res Clin Pract 1991; 14 Suppl.: S79–82
Noury J, Nandeuil A. Comparative three month study of the efficacies of metformin and gliclazide in the treatment of NIDD. Diabete Metab 1991; 17: 209–12
Kramer W, Muller G, Girbig F, et al. Differential interaction of glimepiride and glibenclamide with the beta cell sulfonylrea receptor. II. Photoaffinity labelling of a 65 kDA protein by [3H] glimepiride. Biochim Biophys Acta 1994; 1191: 278–90
Dills DG, Schneider J. Glimepiride/Glyburide Research Group. Clinical evaluation of glimepiride versus glyburide in NIDDM in double-blind comparative study. Horm Metab Res 1996; 28: 426–9
Draeger KE, Wernicke-Panten K, Lomp HJ, et al. Long term treatment of type 2 diabetic patients with the new oral antidiabetic agent glimepiride (Amaryl): a double blind comparison with glibenclamide. Horm Metab Res 1996; 28: 419–25
Dunnig BE. Nateglinide: a glucose sensitive insulinotropic agent that is chemically and pharmacologically distinct from the sulfonylureas. Curr Opin Endocrinol Diabetes 1999; 6 Suppl.: S29–31
Ladriere L, Malaisse-Lagae F, Fuhlendorff J, et al. Effect of antidiabetic agents on the increase in glycemia and insulinemia caused by refeeding in hereditarily diabetic rats. Res Commun Mol Pathol Pharmacol 1997; 97: 53–9
Landgraf R. Meglitinide analogues in the treatment of type 2 diabetes mellitus. Drugs Aging 2000; 17: 411–25
Marbury TC, Runckle JL, Hatorp V, et al. Pharmacokinetics of repaglinide in subjects with renal impairment. Clin Pharmacol Ther 2000: 67: 7–15
Goldberg RB, Einhorn D, Lucas CP, et al. A double blind randomized comparison of meal related glycemic control by repaglinide and glyburide in well controlled type 2 diabetic patients. Diabetes Care 1999; 22: 789–94
Owens DR, Luzio SD, Ismail I, et al. Increased prandial insulin secretion after administration of a single preprandial oral dose of repaglinide in patients with type 2 diabetes. Diabetes Care 2000; 23: 518–23
Wolffenbuttel BH, Nijst L, Sels JP, et al. Effects of a new oral hypoglycemic agent, repaglinide, on metabolic control in sulphonylurea treated patients with NIDDM. Eur J Clin Pharmacol 1993; 45: 113–6
Landgraf R, Bilo HJ, Muller PG. A comparison of repaglinide and glibenclamide in the treatment of type 2 diabetic patients previously treated with sulphonylureas. Eur J Clin Pharmacol 1999; 55: 165–71
Wolffenbuttel BH, Landgraf A. A1-year multicenter randomized double blind comparison of repaglinide and glyburide for the treatment of Type 2 diabetes: Dutch and German Repaglinide Study Group. Diabetes Care 1999; 22: 463–7
Marbury T, Huang WC, Strange P, et al. Repaglinide versus glyburide: a one year comparison trial. Diabetes Res Clin Pract 1999; 43: 155–66
Madsbad S, Kolhovd B, Lager I, et al. Superior glycemie control with repaglinide compared with glipizide in type 2 diabetics. Diabetes 2000; 49: A1514
Moses R, Slobodniuk R, Boyages S, et al. Effect of repaglinide addition to metformin monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 1999; 22: 119–24
Damsbo P, Clauson P, Marbury TC, et al. A double blind randomized comparison of meal related glycemic control by repaglinide and glyburide in well controlled type 2 diabetic patients. Diabetes Care 1999; 22: 789–94
Hanefeld M, Bouter KP, Dickinson S, et al. Rapid and short acting mealtime insulin secretion with nateglinide controls both prandial and mean glycemia. Diabetes Care 2000; 23: 202–7
Hirschberg Y, Karara AH, Pietri AO, et al. Improved control of mealtime glucose excursions with coadministration of nateglinide and metformin. Diabetes Care 2000; 23: 349–53
Dunn CJ, Peters DH. Metformin: a review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes mellitus. Drugs 1995; 49: 721–49
Marena S, Tagliaferro V, Montegrosso G, et al. Metabolic effects of metformin addition to chronic glibenclamide treatment in Type 2 diabetes. Diabete Metab 1994; 20: 15–9
Giugliano D, Quatraro A, Consoli G, et al. Metformin for obese, insulin treated diabetic patients: improvement in glycaemic control and reduction of metabolic risk factors. Eur J Clin Pharmacol 1993; 44: 107–12
Miyazaki Y, Mahankali A, Matsuda M, et al. Improved glycemic control and enhanced insulin sensitivity in Type 2 diabetic subjects treated with pioglitazone. Diabetes Care 2001; 24: 710–9
Aronoff S, Rosenblatt S, Braithwaite S, et al. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with Type 2 diabetes. Diabetes Care 2000; 23: 1605–11
Berms DN, Alter LA, Beckage NJ, et al. Altering the association properties of insulin by amino acid replacement. Protein Eng 1992; 6: 527–33
Howey DC, Bowsher RR, Brunelle RL, et al. [Lys (B28), Pro (B29)] -human insulin: a rapidly absorbed analogue of human insulin. Diabetes 1994; 43: 396–402
Wilde MI, McTavish D. Insulin Lispro: a review of its pharmacological properties and therapeutic use in the management of diabetes mellitus. Drugs 1997; 54: 597–614
Anderson Jr JH, Brunelle RL, Keohane P, et al. Mealtime treatment with insulin analog improves postprandial hyperglycemia and hypoglycemia in patients with non insulin dependent diabetes mellitus: Multicenter Insulin Lispro Study Group. Arch Intern Med 1997; 157: 1249–55
Gale EA. A randomized controlled trial comparing insulin lispro with human soluble insulin in patients with Type 1 diabetes on intensified insulin therapy: the UK Trial Group. Diabet Med 2000; 17: 209–14
Fineberg NS, Fineberg SE, Anderson JH, et al. Immunologic effects of insulin lispro [Lys (B28), Pro (B29) human insulin] in IDDM and NIDDM patients previously treated with insulin. Diabetes 1996; 45: 1750–4
Jovanovic L, Ilic S, Pettit DJ, et al. The metabolic and immunological effects of insulin lispro in gestational diabetes. Diabetes Care 1999; 22: 1422–7
Heinemann L, Heise T, Jorgensen LN, et al. Action profile of the rapid acting insulin analogue: human insulin B28 Asp. Diabet Med 1993; 10: 535–9
Home P, Barriocanal L, Lindholm A. Comparative pharmacokinetics and pharmacodynamics of the novel rapid acting insulin analogue, insulin aspart, in healthy volunteers. Eur J Clin Pharmacol 1999; 55: 199–203
Home PD, Lindholm A, Hylleberg B, et al. UK Insulin Aspart Study Group: improved glycaemic control with insulin aspart -multicentre randomized: double blind cross over trial in type 1 diabetic patients. Diabetes Care 1998; 21: 1904–9
Heise T, Weyer C, Serwas A, et al. Time-action profile of novel premixed preparations of insulin lispro and NPL insulin. Diabetes Care 1998; 21: 800–3
Koivisto VA, Tuominen JA, Ebeling P. Lispro Mix 25 insulin as a premeal therapy in Type 2 diabetic patients. Diabetes Care 1999; 22: 459–62
Roach P, Yue L, Arora V. Humalog Mix 25 Study Group. Improved postprandial glycemic control drug treatment with Humalog Mix25, a novel protamine-based insulin lispro formulation. Diabetes Care 1999; 22: 1258–61
Colombel A, Murat A, Krempf M, et al. Improvement of blood glucose control in Type 1 diabetic patients treated with lispro and multiple NPH injections. Diabet Med 1999; 16: 319–24
Bailey CJ. Novel compounds for NIDDM. Diabetes Rev Int 1996; 5: 9–12
Edwards CM, Stanley SA, Davis R, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001; 281; E155–61
Schmitz O, Nyholm B, Orskov L, et al. Effects of amylin and the amylin agonist pramlintide on glucose metabolism. Diabetes Med 1997; 14Suppl. 2: S19–23
Kong MF, King P, MacDonald IA, et al. Infusion of pramlintide, a human amylin analogue, delays gastric emptying in men with IDDM. Diabetologia 1997; 40: 82–8
Kolterman OG, Gottlieb A, Moyses C, et al. Reduction of postprandial hyperglycemia in subjects with IDDM by intravenous infusion of AC 137, a human amylin analogue. Diabetes Care 1995; 18: 1179–82
Thompson RG, Peterson J, Gotlieb A, et al. Effects of pramlintide, an analog of human amylin, on plasma glucose profiles in patients with IDDM: results of a multicenter trial. Diabetes 1997; 46: 632–6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sudhir, R., Mohan, V. Postprandial Hyperglycemia in Patients with Type 2 Diabetes Mellitus. Mol Diag Ther 1, 105–116 (2002). https://doi.org/10.2165/00024677-200201020-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024677-200201020-00004