Abstract
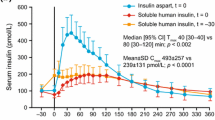
Insulin aspart (NovoLog®, NovoRapid®2), a rapid-acting human insulin analog, provides more rapid absorption than regular human insulin after subcutaneous administration.
In most randomized, nonblind clinical trials in patients with type 1 diabetes mellitus, insulin aspart administered immediately before meals resulted in significantly lower mean glycosylated hemoglobin (HbA1c) levels than regular human insulin (usually administered 30 minutes before a meal). Insulin aspart also significantly improved postprandial glycemic control compared with regular human insulin. The efficacy of insulin aspart was similar to that of insulin lispro when administered to patients with type 1 diabetes mellitus via continuous subcutaneous infusion in a randomized, nonblind trial.
Preliminary data from randomized, nonblind trials suggest insulin aspart had a trend towards lower HbA1c levels compared with regular human insulin in patients with type 2 diabetes mellitus.
Biphasic insulin aspart (30% soluble [rapid-acting] and 70% protamine-bound insulin aspart [BIAsp30]) [NovoLog® Mix 70/30, NovoMix® 302] generally provided significantly better postprandial glucose control than a similar mixture of biphasic regular human insulin (BHI30) in a randomized, nonblind trial in patients with type 1 or 2 diabetes mellitus. However, the long-term efficacy of BIAsp30 was similar to that of BHI30 after 2 years in a randomized, nonblind trial in patients with type 2 diabetes mellitus.
Patients with type 1 or 2 diabetes mellitus reported greater treatment satisfaction with insulin aspart or BIAsp30 than with regular human insulin or BHI30.
The overall incidence of hypoglycemia with insulin aspart was lower than, or similar to, that of regular human insulin. Moreover, insulin aspart tended to be associated with a lower occurrence of nocturnal hypoglycemia and severe hypoglycemic events than regular human insulin.
Conclusion: The standard preparation of insulin aspart has the potential to better mimic the physiological response to meals than regular human insulin. Insulin aspart when combined with a suitable basal insulin improved overall glycemie control and led to a similar or lower number of hypoglycemie episodes compared with a similar regular human insulin regimen. Insulin aspart was generally as effective and well tolerated as insulin lispro when administered by continuous subcutaneous infusion in a single comparative trial. The efficacy of biphasic insulin aspart has been documented in a small number of trials. Both insulin aspart and biphasic insulin aspart provide for flexible and convenient administration. Insulin aspart is now well established as an effective and convenient means of providing glycemie control which offers clinical and practical advantages over regular human insulin.
Similar content being viewed by others
References
Whittingham JL, Edwards DJ, Antson AA, et al. Interactions of phenol and m-cresol in the insulin hexamer, and their effect on the association properties for B28 Pro→Asp insulin analogues. Biochemistry 1998; 37: 11516–23
Gammeltoft S, Hansen BF, Dideriksen L, et al. Insulin aspart: a novel rapid-acting human insulin analogue. Expert Opin Invest Drugs 1999; 8(9): 1431–41
Drejer K, Kruse V, Larsen UD, et al. Receptor binding and tyrosine kinase activation by insulin analogues with extreme affinities studied in human hepatoma HepG2 cells. Diabetes 1991 Nov; 40: 1488–95
Volund A, Brange J, Drejer K, et al. In vitro and in vivo potency of insulin analogues designed for clinical use. Diabet Med 1991 Nov; 8(9): 839–47
Kurtzhals P, Schäffer L, Drejer K, et al. Insulin receptor tyrosine kinase activation by insulin aspart and insulin analogues with a wide range of receptor binding affinities [abstract no. 667]. Diabetologia 1999 Aug; 42Suppl. 1: A179
Kurtzhals P, Schäffer L, Sorensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000 Jun; 49(6): 999–1005
Trüb T, Hey A, Sorensen AR, et al. Growth promoting activity of insulin aspart and other insulin analogues [abstract no. 666]. Diabetologia 1999 Aug; 42Suppl. 1: 179
Frier BM, Ewing FME, Lindholm A, et al. Symptomatic and counterregulatory hormonal responses to acute hypoglycaemia induced by insulin aspart and soluble human insulin in type 1 diabetes. Diabetes Metab Res Rev 2000 Jul; 16(4): 262–8
Heller SR, Robinson RTCE, Harris ND, et al. Soluble insulin and the rapid-acting analogue insulin aspart have similar effects upon ventricular repolarization [abstract no. 891]. Diabetologia 1999 Aug; 42Suppl. 1: 237
Heinemann L, Heise T, Jorgensen LN, et al. Action profile of the rapid acting insulin analogue: human insulin B28Asp. Diabet Med 1993; 10: 535–9
Heinemann L, Kapitza C, Starke AA, et al. Time-action profile of the insulin analogue B28Asp. Diabet Med 1996 Jul; 13(7): 683–4
Heinemann L, Weyer C, Rave K, et al. Comparison of the time-action profiles of U40- and U100-regular human insulin and the rapid-acting insulin analogue B28 Asp. Exp Clin Endocrinol Diabetes 1997; 105: 140–4
Heinemann L, Weyer C, Rauhaus M, et al. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care 1998 Nov; 21(11): 1910–4
Mudaliar SR, Lindberg FA, Joyce M, et al. Insulin aspart (B28 asp-insulin): a fast-acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care 1999 Sep; 22(9): 1501–6
Home PD, Barriocanal L, Lindholm A. Comparative pharmacokinetics and pharmacodynamics of the novel rapid-acting insulin analogue, insulin aspart, in healthy volunteers. Eur J Clin Pharmacol 1999 May; 55(3): 199–203
Kaku K, Matsuda M, Urae A, et al. Pharmacokinetics and pharmacodynamics of insulin aspart, a rapid-acting analog of human insulin, in healthy Japanese volunteers. Diabetes Res Clin Pract 2000 Aug; 49: 119–26
Kang S, Brange J, Burch A, et al. Absorption kinetics and action profiles of subcutaneously administered insulin analogues (AspB9GluB27, AspB10, AspB28) in healthy subjects. Diabetes Care 1991 Nov; 14(11): 1057–65
Lindholm A, McEwen J, Riis AP. Improved postprandial glycemic control with insulin aspart: a randomized double-blind cross-over trial in type 1 diabetes. Diabetes Care 1999 May; 22(5): 801–5
Kang S, Creagh FM, Peters JR, et al. Comparison of subcutaneous soluble human insulin and insulin analogues (AspB9, GluB27; AspB10, AspB28) on meal-related plasma glucose excursions in type I diabetic subjects. Diabetes Care 1991 Jul; 14(7): 571–7
Lutterman JA, Pijpers E, Netten PM, et al. Glycaemic control in IDDM patients during one day with injection of human insulin or the insulin analogues insulin X14 and insulin X14 (+Zn). In: Berger M, Gries F, editors. Frontiers in insulin pharmacology: international symposium, Hamburg. Stuttgart: Thieme Medical Publishers, 1993: 102–9
Rosenfalck AM, Thorsby P, Kjems L, et al. Improved postprandial glycaemic control with insulin aspart in type 2 diabetic patients treated with insulin. Acta Diabetol 2000 Mar; 37(1): 41–6
Perriello G, Avogaro A, Emanuele B, et al. Superior meal-time glucose control with insulin aspart (NovoLog®) compared with human insulin in both normalweight and overweight people with type 2 diabetes — a randomized, stratified, double-blind, crossover trial [abstract no. 452-P]. Diabetes 2002 Jun; 51Suppl. 2: A111
Mortensen HB, Lindholm A, Olsen BS, et al. Rapid appearance and onset of action of insulin aspart in paediatric subjects with type 1 diabetes. Eur J Pediatr 2000 Jul; 159(7): 483–8
Jacobsen LV, Sogaard B, Riis A. Pharmacokinetics and pharmacodynamics of a premixed formulation of soluble and protamine-retarded insulin aspart. Eur J Clin Pharmacol 2000 Aug; 56(5): 399–403
Weyer C, Heise T, Heinemann L, et al. Insulin aspart in a 30/70 premixed formulation: pharmacodynamic properties of a rapid-acting insulin analog in stable mixture. Diabetes Care 1997 Oct; 20(10): 1612–4
Hermansen K, Vaaler S, Madsbad S, et al. Postprandial glycemic control with biphasic insulin aspart in patients with type 1 diabetes. Metabolism 2002 Jul; 51(7): 896–900
McSorley PT, Bell PM, Jacobsen LV, et al. Twice-daily biphasic insulin aspart 30 versus biphasic human insulin 30: a double-blind crossover study in adults with type 2 diabetes mellitus. Clin Ther 2002; 24(4): 530–9
Hermansen K, Colombo M, Storgaard H, et al. Improved postprandial glycemic control with biphasic insulin aspart relative to biphasic insulin lispro and biphasic human insulin in patients with type 2 diabetes. Diabetes Care 2002 May; 25(5): 883–8
Plank J, Wutte A, Goerzer E, et al. A direct comparison of insulin analogs aspart and lispro in type 1 diabetic patients [abstract no. 213-OR]. Diabetes 2002 Jun; 51Suppl. 2: A52
Brunner GA, Hirschberger S, Sendlhofer G, et al. Post-prandial administration of the insulin analogue insulin aspart in patients with type 1 diabetes mellitus. Diabet Med 2000 May; 17(5): 371–5
Gallagher A, Home PD. The effect of insulin aspart in post-prandial lipid profile in type 2 diabetes [abstract no. 1932-PO]. Diabetes 2002 Jun; 51Suppl. 2: A470
Hamilton-Wessler M, Buchanan T, Hathout E, et al. Time-action profile for intravenously infused insulin aspart and insulin lispro in type 1 diabetic subjects [abstract no. 403-P]. Diabetes 2002 Jun; 51(Suppl. 2): A99
Lindholm A, Jacobsen LV. Clinical pharmacokinetics and pharmacodynamics of insulin aspart. Clin Pharmacokinet 2001; 40(9): 641–59
Hedman CA, Lindstrom T, Arnqvist HJ. Direct comparison of insulin lispro and aspart shows small differences in plasma insulin profiles after subcutaneous injection in type 1 diabetes [letter]. Diabetes Care 2001 Jun; 24(6): 1120–1
Lyness W, Tyler JF, Lawrence A. Renal impairment does not affect insulin aspart pharmacokinetics in type 1 diabetes [abstract no. 842-PO]. Diabetes 2001 Jun; 50Suppl. 2: A441–2
Lyness W, Tyler J, Lawrence A. Pharmacokinetics of the rapid-acting insulinanalog, insulin aspart, is independent of hepatic function [abstract no. 1843-PO]. Diabetes 2001 Jun; 50Suppl. 2: A442
Lyness W, Tyler J, Lawrence A. BMI does not affect insulin aspart pharmacokinetics in type I diabetes [abstract no. 499-P]. Diabetes 2001 Jun; 50Suppl. 2: A124
Home PD, Lindholm A, Hylleberg B, et al. Improved glycemie control with insulin aspart: a multicenter randomized double-blind crossover trial in type 1 diabetic patients. Diabetes Care 1998 Nov; 21(11): 1904–9
Home PD, Lindholm A, Riis A. Insulin aspart vs. human insulin in the management of long-term blood glucose control in type 1 diabetes mellitus: a randomized controlled trial. Diabet Med 2000 Nov; 17(11): 762–70
Raskin P, Guthrie RA, Leiter L, et al. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care 2000 May; 23(5): 583–8
Tamás G, Marre M, Astorga R, et al. Glycaemic control in type 1 diabetic patients using optimised insulin aspart or human insulin in a randomised multinational study. Diabetes Res Clin Pract 2001 Nov; 54: 105–14
Tsalikian E. Insulin aspart in pediatrie and adolescent patients with type 1 diabetes [abstract no. 767]. Diabetologia 2000 Aug; 43Suppl. 1: 200
DeVries JH, Lindholm A, Heine RJ, et al. A randomized trial of insulin aspart with intensified basal NPH insulin supplementation in type 1 diabetes [abstract no. 801]. Diabetologia 2001; 44Suppl. 1: 209
Bretzel RG, Medding J, Hirschberger S, et al. Insulin aspart efficacy and safety compared to human soluble insulin and human premix insulin (30/70) in patients with type 2 diabetes mellitus [abstract no. 804]. Diabetologia 2001; 44Suppl. 1: 209
McGill J, Raskin P, Kilo C, et al. Human insulin analog, insulin aspart, is a mealtime insulin comparable to human insulin in type 2 diabetes [abstract no. 894]. Diabetologia 1999 Aug; 42Suppl. 1: A238
Amiel S, Home PD, Jacobsen JL, et al. Insulin aspart safe for long-term treatment [abstract no. 802]. Diabetologia 2001; 44Suppl. 1: A209
Bode B, Weinstein R, Bell D, et al. Comparison of insulin aspart with buffered regular insulin and insulin lispro in continuous subcutaneous insulin infusion: a randomized study in type 1 diabetes. Diabetes Care 2002 Mar; 25(3): 439–44
Danne T, Aaman J, Schober E, et al. Preprandial vs. postprandial insulin aspart treatment in type 1 diabetic children and adolescents [abstract no. 986]. Diabetologia 2001; 44Suppl. 1: 257
Bretzel RG, Hirschberger S, Medding J, et al. A multi-centre efficacy and safety comparison of insulin aspart, human soluble insulin and human premix insulin (70/30) in patients with type 2 diabetes mellitus [abstract no. 379-P]. Diabetes 2002 Jun; 51Suppl. 2: A93
Boehm BO, Home PD, Behrend C, et al. Premixed insulin aspart 30 vs. premixed human insulin 30/70 twice daily: a randomized trial in type 1 and type 2 diabetic patients. Diabet Med 2002; 19: 393–9
Boehm B, Home P, Kamp N, et al. Biphasic insulin aspart and biphasic human insulin compared in type 2 diabetic subjects [abstract no. 808]. Diabetologia 2001; 44Suppl. 1:210
Warren ML, Conway MJ, Klaff LJ, et al. Clinical experience with postprandial dosing of NovoLog Mix 70/30 in elderly subjects with type 2 diabetes [abstract no. 1999-PO]. Diabetes 2002 Jun; 51Suppl. 2: A486
Kilo C, Mezitis NH, Nadeau DA, et al. Dinnertime Novolog mix 70/30 in combination with metformin: a combination to use? [abstract no. 527-P] Diabetes 2002 Jun; 51Suppl. 2: A130
Kvapil M, Mikolajczyk-Swatko A, Ostergaard AH, et a1. Biphasic insulin aspart 30 combined with metformin provides better glycemie control in poorly controlled type 2 diabetic patients [abstract no. 424-P]. Diabetes 2002 Jun; 51Suppl. 2: A104
Bott U, Ebrahim S, Hirschberger S, et al. Effect of the insulin analogue insulin aspart on quality-of-life and treatment satisfaction in type 1 diabetic patients [abstract no. 893]. Diabetologia 1999 Aug; 42Suppl. 1: 237
Home PD, Boehm BO, Bott U, et al. Treatment with premixed insulin aspart 30 in type 1 and type 2 diabetes: a randomized 12-week comparison [abstract no. P220]. Diabetes Res Clin Pract 2000 Sep; 50Suppl. 1: S37
Colagiuri S, Heller S, Vaaler S, et al. Insulin aspart reduces the frequency of nocturnal hypoglycaemia in patients with type 1 diabetes [abstract no. 805]. Diabetologia 2001; 44Suppl. 1: 210
Lindholm A, Andersen HF, Gall M. Safety profile of insulin aspart [abstract no. P110]. Diabet Med 2000 Mar; 17Suppl. 1: 62
NovoNordisk. NovoLog® Clinical Overview. (Data on file). 2001 Jul
Lindholm A, Jensen LB, Home PD, et al. Immune responses to insulin aspart and biphasic insulin aspart in people with type 1 and type 2 diabetes. Diabetes Care 2002 May; 25(5): 876–82
Novo Nordisk. NovoRapid®: summary of product characteristics [online]. Available from URL: http://www.novonordisk.co.uk/view.asp?ID=70. [Accessed 2002 Aug 13]
Novo Nordisk. Prescribing information: NovoLog®. Insulin aspart (rDNA origin) injection [online]. Available from URL:://www.novolog.com/physician/content/pe_pdfs/ PhysicianInsert.pdf. URL [Accessed 2002 Aug 13]
Novo Nordisk. NovoMix30® Penfill® and FlexPen®: summary of product characteristics [online]. Available from URL: http://www.novonordisk.co.uk/ view.asp?ID=1496. [Accessed 2002 Aug 13]
Novo Nordisk. Prescribing information: NovoLog Mix 70/30®. 2002 Jul 13
Novo Nordisk. NovoRapid® and NovoMix30®: treatment guidelines for adults [online]. Available from URL: http://www.novomix30.com/novomix/treat-mentOl.asp. [Accessed 2002 Aug 13]
Author information
Authors and Affiliations
Corresponding author
Additional information
This Spotlight is derived from abstract and summary text of an Adis Drug Evaluation originally published in full in Drugs 2002; 62 (13): 1945–1981
Use of tradenames is for product identification purposes only and does not imply endorsement.
Rights and permissions
About this article
Cite this article
Chapman, T.M., Noble, S. & Goa, K.L. Spotlight on Insulin Aspart in Type 1 and 2 Diabetes Mellitus. Mol Diag Ther 2, 71–76 (2003). https://doi.org/10.2165/00024677-200302010-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024677-200302010-00006