Abstract
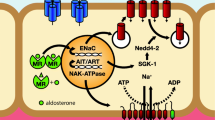
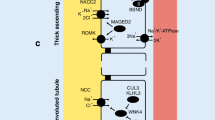
The epithelial sodium channel (ENaC) has a central role in sodium transport across membranes. It is expressed on the apical cell surface of renal tubular epithelia and also on other aldosterone-responsive epithelial cells. In the kidney, ENaC contributes to the regulation of blood pressure via changes in sodium balance and blood volume. Rare monogenetic disorders associated with hypertension have been described, such as Liddle syndrome, which gives rise to increased sodium reabsorption in the kidney via increased ENaC activity. There are many other variants in the genes encoding ENaC subunits, some of which occur with sufficient frequency as to be termed polymorphic variants. The Thr594Met polymorphism of the ENaC β-subunit gene SCNN1B occurs exclusively in Black individuals, with a frequency of 6–8% in those with hypertension. It increases cAMP mediated ENaC sodium current in affected B lymphocytes, and has been associated with hypertension in a Black South London population.
There is preliminary evidence that amiloride is effective as monotherapy in hypertensive individuals with the Thr594Met polymorphism and in patients with resistant hypertension, who have evidence of increased amiloride-sensitive sodium channel activity. If these preliminary studies are corroborated in larger studies, then amiloride may provide an important new strategy for blood pressure control in selected individuals.



Similar content being viewed by others
References
Shimkets RA, Warnock DG, Bositis CM, et al. Liddle’s syndrome: heritable human hypertension caused by mutations in the β subunit of the epithelial sodium channel. Cell 1994 Nov 4; 79(3): 407–14
Chang SS, Grunder S, Hanukoglu A, et al. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic alkalosis, psuedohy-poaldosteronism type 1. Nat Genet 1996 Mar; 12(3): 248–53
Hummler E, Barker P, Talbot C, et al. A mouse model for the renal salt-wasting syndrome pseudohypoaldosteronism. Proc Natl Acad Sci U S A 1997 Oct; 94(21): 11710–5
McDonald FJ, Yang B, Hrstka RF, et al. Disruption of the beta subunit of the epithelial Na+ channel in mice: hyperkalemia and neonatal death associated with a pseudohypoaldosteronism phenotype. Proc Natl Acad Sci U S A 1999 Feb 16; 96(4): 1727–31
Gombos EA, Freis ED, Moghadam A. Effects of MK-870 in normal subjects and hypertensive patients. N Engl J Med 1966 Dec; 275(22): 1215–20
Millar JA, Fraser R, Mason P, et al. Metabolic effects of high dose amiloride and spironolactone: a comparative study in normal subjects. Br J Clin Pharmacol 1984 Sep; 18(3): 369–75
Pratt JH, Eckert GJ, Newman S, et al. Blood pressure responses to small doses of amiloride and spironolactone in normotensive subjects. Hypertension 2001 Nov; 38(5): 1124–9
Baker EH, Ireson NJ, Carney C, et al. Transepithelial sodium absorption is increased in people of African origin. Hypertension 2001 Jul; 38(1): 76–80
Ambrosius WT, Bloem LJ, Zhou L, et al. Genetic variants in the epithelial sodium channel in relation to aldosterone and potassium excretion and risk for hypertension. Hypertension 1999 Oct; 34 (4 Pt 1): 631–7
Persu A, Barbry P, Bassilana F, et al. Genetic analysis of the beta subunit of the epithelial Na+ channel in essential hypertension. J Hypertens 1998 Jul; 32(1): 129–37
Persu A, Coscoy S, Houot AM, et al. Polymorphisms of the gamma-subunit of the epithelial Na+ channel in essential hypertension. Hypertension 1999 May; 17(5): 639–45
Rayner BL, Owen EP, King JA, et al. A new mutation, R563Q, of the beta subunit of the epithelial sodium channel associated with low-renin, low-aldosterone hypertension. J Hypertens 2003 May; 21(5): 921–6
Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 2002 Jul; 82(3): 735–67
Canessa CM, Schild L, Beull G, et al. Amiloride-sensitive Na+ channel is made of three homologous subunits. Nature 1994 Feb 3; 367(6462): 463–7
Renard S, Lingueglia E, Voilley N, et al. Biochemical analysis of the membrane topology of the amiloride-sensitive Na+ channel. J Biol Chem 1994 Apr 29; 269(17): 12981–6
Rossier BC, Pradervand S, Schild L, et al. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol 2002; 64: 877–97
Schild L, Schneeberger E, Gautschi I, et al. Identification of amino acid residues in the alpha, beta, and gamma subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol 1997 Jan; 109(1): 15–26
Firsov D, Gautschi I, Merillat AM, et al. The heterotetrameric architecture of the epithelial sodium channel (ENaC). EMBO J 1998 Jan 15; 17(2): 344–52
Kosari F, Sheng S, Li J, et al. Subunit stoichiometry of the epithelial sodium channel. J Biol Chem 1998 May 29; 273(22): 13469–74
Snyder PM, Cheng C, Prince LS, et al. Electrophysiological and biochemical evidence that DEG/ENaC cation channels are composed of nine subunits. J Biol Chem 1999 Jan 9; 273(2): 681–4
Eskandari S, Snyder PM, Kreman M, et al. Number of subunits comprising the epithelial sodium channel. J Biol Chem 1999 Sep 17; 273(38): 27281–6
Sheng S, Li J, McNulty KA, et al. Epithelial sodium channel pore region: structure and role in gating. J Biol Chem 2001 Jan 12; 276(2): 1326–34
McNicholas CM, Canessa CM. Diversity of channels generated by different combinations of epithelial sodium channel subunits. J Gen Physiol 1997 Jun; 109(6): 681–92
Kellenberger S, Hoffman-Pochon N, Gautschi I, et al. On the molecular basis of ion permeation in the epithelial Na+ channel. J Gen Physiol 1999 Jul; 114(1): 13–30
Kellenberger S, Gautschi I, Schild L. A single point mutation in the pore region of the epithelial Na+ channel changes ion selectivity by modifying molecular sieving. Proc Natl Acad Sci U S A 1999 Mar 30; 96(7): 4170–5
Staub O, Abriel H, Plant P, et al. Regulation of the epithelial sodium channel by Nedd4 and ubiquitinisation. Kidney Int 2000 Mar; 57(3): 809–15
Gormley K, Dong Y, Sagnella GA. Regulation of the epithelial sodium channel by accessory proteins. Biochem J 2003 Apr 1; 371 (Pt 1): 1–14
Lifton RP. Molecular genetics of human blood pressure variation. Science 1996 May 3; 272(5262): 676–80
Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 2001 Feb 23; 104(4): 545–56
Liddle GW, Bledsoe T, Coppage WS. A familial renal disorder simulating primary aldosteronism but with negligable aldosterone secretion. Trans Assoc Am Physicians 1963; 76: 199–213
Botero-Velez M, Curtis JJ, Warnock DG. Brief report: Liddle’s Syndrome revisited: a disorder of sodium reabsorption in the distal tubule. N Engl J Med 1994 Jan 20; 330(3): 178–81
Gadallah MF, Abreo K, Work J. Liddle’s syndrome, an under recognised entity: a report of four cases, including the first report in black individuals. Am J Kidney Dis 1995 Jun; 25(6): 829–35
Hansson JH, Nelson-Williams C, Suzuki H, et al. Hypertension caused by a truncated epithelial sodium channel y subunit: genetic heterogeneity of Liddle syndrome. Nat Genet 1995 Sep; 11(1): 76–82
Tamura H, Schild L, Enomoto N, et al. Liddle disease caused by a missense mutation of β subunit of the epithelial sodium channel gene. J Clin Invest 1996 Apr 1; 97(7): 1780–4
Staub O, Dho S, Henry P, et al. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J 1996 May 15; 15(10): 2371–80
Snyder PM, Price MP, McDonald FJ, et al. Mechanism by which Liddle’s syndrome mutations increase activity of a human epithelial Na+ channel. Cell 1995 Dec 15; 83(6): 969–78
Saxena A, Hanukoglu I, Saxena D, et al. Novel mutations responsible for autosomal recessive multisystem psuedohypoaldosteronism and sequence variants in epithelial sodium channel alpha-, beta- and gamma-subunit genes. J Clin Metab 2002 Jul; 87(7): 3344–50
Baker EH, Dong YB, Sagnella GA, et al. Association of hypertension with T594M mutation in β subunit of epithelial sodium channels in black people resident in London. Lancet 1998 May 9; 351(9113): 1388–92
Dong YB, Zhu HD, Baker EH, et al. T594M and G442V polymorphisms of the sodium channel β-subunit and hypertension in a black population. J Hum Hypertens 2001 Jun; 15(6): 425–30
Matsubara M, Ohkubo T, Michimata M, et al. Japanese individuals do not harbour the T594M mutation but do have the P592S mutation in the C-terminus of the β-subunit of the epithelial sodium channel: the Ohasama Study. J Hypertens 2000; 18: 861–6
Sugiyama T, Kato N, Ishinaga Y, et al. Evaluation of selected polymorphism s of the Mendelian hypertensive disease genes and the Japanese population. Hypertens Res 2001 Sep; 24(5): 515–21
Su YR, Rutkowski MP, Klanke CA, et al. A novel variant of the β-subunit of the amiloride-sensitive sodium channel in African Americans. J Am Soc Nephrol 1996 Dec; 7(12): 2543–9
Cui Y, Su YR, Rutkowski MP, et al. Loss of protein kinase C inhibition in the β-T594M variant of the amiloride-sensitive Na+ channel. Proc Natl Acad Sci U S A 1997 Sep 2; 94(18): 9962–6
Su YR, Rutkowski MP, Klanke CA, et al. A novel variant of the β-subunit of the amiloride-sensitive sodium channel in African Americans. J Am Soc Nephrol 1996 Dec; 7(12): 2543–9
Bubien JK, Warnock DG. Amiloride-sensitive sodium conductance in human B lymphoid cells. Am J Physiol 1993 Oct; 265 (4 Pt 1): C1175–83
Bubien JK, Ismailov II, Berdiev BK, et al. Liddle’s disease: abnormal regulation of amiloride-sensitive Na+ channels by beta-subunit mutation. Am J Physiol 1996 Jan; 270 (1 Pt 1): C208–13
Bubien JK, Watson B, Khan MA, et al. Expression and regulation of normal and polymorphic epithelial sodium channel by human lymphocytes. J Biol Chem 2001 Mar 16; 276(11): 8557–66
Oh Y, Warnock DG. Expression of the amiloride-sensitive sodium channel beta subunit gene in human B lymphocytes. J Am Soc Nephrol 1997 Jan; 8(1): 126–9
Ottaviani E, Franchini A, Mandrioli M, et al. Amiloride-sensitive epithelial sodium channel subunits are expressed in human and mussel immunocytes. Dev Comp Immunol 2002 Jun; 26(5): 395–402
Tiago AD, Nkeh B, Candy GP, et al. Association study of eight candidate genes with renin status in mild-to-moderate hypertension in patients of African ancestry. Cardiovasc J S Afr 2001 Apr-May; 12(2): 75–80
Findling JW, Raff H, Hansson JH, et al. Liddle’s syndrome: Prospective genetic screening and suppressed aldosterone secretion in an extended kindred. J Clin Endocrinol Metab 1997 Apr; 82(4): 1071–4
Blackwood AM, Baker EH, Dong YB, et al. The T594M mutation of the β-subunit of the amiloride sensitive sodium channel is associated with increased levels of urinary calcium excretion [abstract]. Hypertension 1998 Oct; 32: 793
Baker EH, Duggal A, Dong Y, et al. Amiloride, a specific drug for hypertension in black people with T594M variant? Hypertension 2002 Jul; 40(1): 13–7
Multicentre Diuretic Cooperative Study Group. Multiclinic comparison of amiloride, hydrochlorothiazide and hydrochlorothiazide plus amiloride in essential hypertension. Arch Intern Med 1981 Mar; 141(4): 482–6
Katzman PL, Henningsen NC, Hulthen UL. Amiloride compared with nitrendipine in treatment of essential hypertension. J Hum Hypertens 1988 Oct; 2(3): 147–51
Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men: a comparison of six antihypertensive agents with placebo: the Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 1993 Apr; 328(13): 914–21
He FJ, Markandu ND, Sagnella GA, et al. Importance of the renin system in determining blood pressure fall with salt restriction in black and white hypertensives. Hypertension 1998 Nov; 32(5): 820–4
Lim PO, Dow E, Brennan G, et al. High prevalence of primary aldosteronism in the Tayside hypertension clinic. J Hum Hypertens 2000 May; 14(5): 311–5
Carter AR, Zhou ZH, Calhoun DA, et al. Hyperactive ENaC identifies hypertensive individuals amenable to amiloride therapy. Am J Physiol Cell Physiol 2001 Nov; 281(5): C1413–21
Acknowledgments
This work was funded by the British Heart Foundation (BHF). There are no conflicts of interest for either Dr P.A. Swift or Professor G.A. MacGregor.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Swift, P.A., MacGregor, G.A. The Epithelial Sodium Channel in Hypertension. Am J Pharmacogenomics 4, 161–168 (2004). https://doi.org/10.2165/00129785-200404030-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00129785-200404030-00003