Abstract
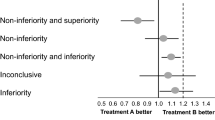
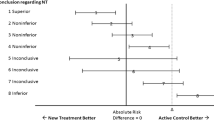
There are some substantial ways in which the current approach to establishing an evidence base for a treatment effect works against complementary and alternative medicine (CAM) research. The standard statistical method in comparative trials is the null hypothesis test, which requires a decision that the ‘no treatment effect’ hypothesis is confirmed, or it is rejected. While this approach is appropriate for evidence-based medicine, the requirement for a decisive result drives sample sizes upward, or leaves small trials subject to being criticised for being underpowered. Employing the fundamental theory of hypothesis tests, as developed by Neyman and Pearson, it is possible to define a ‘separation test’ that avoids this problem, by invoking a different inferential rationale. The purpose of a separation test is to find an indication that further research is justified, or that it is not. This change in strategy can considerably lower the required sample size. Since many CAM comparative trials are in early phases of research, where both budgetary and safety considerations argue for a small sample size, separation tests may be especially of interest to CAM researchers. There is, therefore, an opportunity for evidence-based medicine to generate a new kind of study, in which the purpose is to assess whether it is worthwhile to pursue research on an alternative treatment, rather than to determine whether it has been proved effective.







Similar content being viewed by others
References
Neyman J, Pearson ES. On the problem of the most efficient tests of statistical hypotheses. Philos Trans R Soc Lond A 1933; 236: 333–80
Alfano A, Taylor AG, Foresman PA, et al. Static magnetic fields for treatment of fibromyalgia: a randomized controlled trial. J Altern Complement Med 2001; 7(1): 53–64
Cohen J. Statistical power analysis for the behavioral sciences. Hillsdate (NJ): Lawrence Erlbaum, 1987
Okvat HA, Oz MC, Ting W, et al. Massage therapy for patients undergoing cardiac catheterization. Altern Ther Health Med 2002; 8(3): 68–75
Smith MC, Reeder F, Daniel L, et al. Outcomes of touch therapies during bone marrow transplant. Altern Ther Health Med 2003; 9(1): 40–9
Wellek S. Testing statistical hypotheses of equivalence. London: Chapman & Hall, 2002
Acknowledgements
The authors have provided no information on sources of funding or on conflicts of interest directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aickin, M. Separation Tests for Early-Phase Complementary and Alternative Medicine Comparative Trials. Evid-Based-Integrative-Med 1, 225–231 (2004). https://doi.org/10.2165/01197065-200401040-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/01197065-200401040-00001