Ethanol-Induced Hepatotoxicity and Alcohol Metabolism Regulation by GABA-Enriched Fermented Smilax china Root Extract in Rats
Abstract
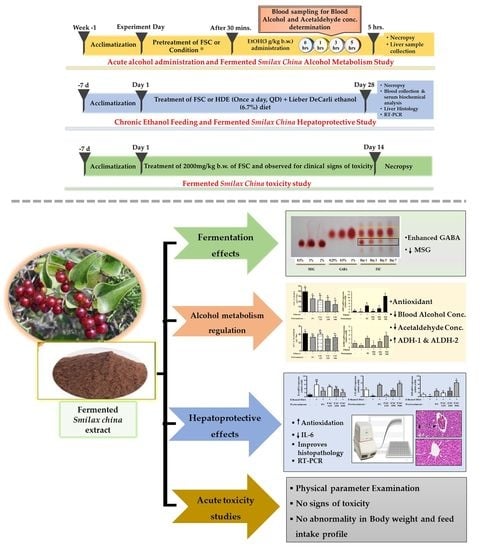
:1. Introduction
2. Materials and Methods
2.1. Extract Preparation and Analysis
2.1.1. Preparation of FSC
2.1.2. Quantification of GABA Content
2.2. Animals and Experimental Design
2.2.1. Animals
2.2.2. Experimental Design for Alcohol Metabolism after Acute Ethanol Administration
2.2.3. Experimental Design
2.2.4. Alcohol and Acetaldehyde Concentration in Serum
2.2.5. Measurement of Liver Biomarkers in Serum
2.2.6. Histopathology
2.2.7. Total RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.3. Acute Oral Toxicity Test
2.4. Statistical Analysis
3. Results
3.1. Fermentation Effects on GABA Content
3.2. Effect of FSC on Alcohol Metabolism
3.2.1. Effect of FSC on Blood Concentration of Alcohol and Acetaldehyde
3.2.2. Effect of FSC on Alcohol-Metabolizing Enzymes of Liver
3.3. Effect of FSC on Chronic Alcohol-Induced Hepatic Damage
3.3.1. Effect of FSC on Serum Biochemical Parameters
3.3.2. Histopathology
3.3.3. Effect of FSC on the Expression of Lipid Metabolism-Related Genes in Rat Liver
3.3.4. Effect of FSC on the Gene Expression of Anti-Inflammatory and Antioxidant Enzymes
3.4. In Vivo Acute Oral Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomon, B.; Battu, R. Antihepatotoxic activity of Smilax China roots on CCL4 induced hepatic damage in rats. Int. J. Pharm. Sci. 2012, 4, 494–496. [Google Scholar]
- Park, N.-H.; Lee, S.-J.; Mechesso, A.F.; Boby, N.; Yixian, Q.; Yoon, W.-K.; Lee, S.-P.; Lee, J.-S.; Park, S.-C. Hepatoprotective effects of gamma-aminobutyric acid-enriched fermented Hovenia dulcis extract on ethanol-induced liver injury in mice. BMC Complement. Med. Ther. 2020, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.W.; Sun, C.; Lay, C. Alcohol hangover headache. Headache 2007, 47, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Prat, G.; Adan, A.; Sanchez-Turet, M. Alcohol hangover: A critical review of explanatory factors. Hum. Psychopharmacol. 2009, 24, 259–267. [Google Scholar] [CrossRef]
- Boby, N.; Abbas, M.A.; Lee, E.-B.; Im, Z.-E.; Hsu, W.H.; Park, S.-C. Protective Effect of Pyrus ussuriensis Maxim. Extract against Ethanol-Induced Gastritis in Rats. Antioxidants 2021, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Mohd Ali, N.; Mohd Yusof, H.; Long, K.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Koh, S.P.; Abdullah, M.P.; Alitheen, N.B. Antioxidant and hepatoprotective effect of aqueous extract of germinated and fermented mung bean on ethanol-mediated liver damage. Biomed. Res. Int. 2013, 2013, 693613. [Google Scholar] [CrossRef]
- Azam, F.; Sheikh, N.; Ali, G.; Tayyeb, A. Fagonia indica repairs hepatic damage through expression regulation of toll-like receptors in a liver injury model. J. Immunol. Res. 2018, 2018, 7967135. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C. Antioxidant capacity of flavanols and gallate esters: Pulse radiolysis studies. Free Radic. Bio. Med. 1999, 27, 1413–1426. [Google Scholar] [CrossRef]
- Kim, K.M.; Suh, J.W.; Yang, S.H.; Kim, B.R.; Park, T.S.; Shim, S.M. Smilax China root extract detoxifies nicotine by reducing reactive oxygen species and inducing CYP2A6. J. Food Sci. 2014, 79, H2132–H2139. [Google Scholar] [CrossRef]
- Saravanakumar, S.; Felicia, C.; Sundarapandian, S. Phytochemical screening of the methanol extract of root tuber of smilax China. Int. J. Pharmacogn. Phytochem. Res. 2015, 6, 963–966. [Google Scholar]
- Feng, F.; Liu, W.-Y.; Chen, Y.-S.; Liu, J.-H.; Zhao, S.-X. Flavonoids and stilbenes from Smilax China. J. China Pharm. Univ. 2003, 34, 119–121. [Google Scholar]
- Shao, B.; Guo, H.; Guo, D. Flavonoids and stilbenes from Smilax China. Chin. Tradit. Herb. Drugs. 2009, 40, 1700–1703. [Google Scholar]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Tremendous health benefits and clinical aspects of Smilax China. Afr. J. Pharm. Pharmacol. 2019, 13, 253–258. [Google Scholar]
- Li, Y.L.; Gan, G.P.; Zhang, H.Z.; Wu, H.Z.; Li, C.L.; Huang, Y.P.; Liu, Y.W.; Liu, J.W. A flavonoid glycoside isolated from Smilax China L. rhizome in vitro anticancer effects on human cancer cell lines. J. Ethnopharmacol. 2007, 113, 115–124. [Google Scholar] [CrossRef]
- Lee, S.E.; Ju, E.M.; Kim, J.H. Free radical scavenging and antioxidant enzyme fortifying activities of extracts from Smilax China root. Exp. Mol. Med. 2001, 33, 263–268. [Google Scholar] [CrossRef]
- Kang, Y.H.; Kim, K.K.; Kim, D.J.; Choe, M. Antiobesity effects of the water-soluble fraction of the ethanol extract of Smilax China L. leaf in 3T3-L1 adipocytes. Nut. Res. Pract. 2015, 9, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.-K.; Lee, J.-H.; Kim, H.-S.; Lee, C.-K.; Lee, S.-C. Antioxidant and antimicrobial activities of Smilax China L. leaf extracts. Food Sci. Biotechnol. 2012, 21, 1723–1727. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Morse, A.C.; Schulteis, G.; Holloway, F.A.; Koob, G.F. Conditioned place aversion to the “hangover” phase of acute ethanol administration in the rat. Alcohol 2000, 22, 19–24. [Google Scholar] [CrossRef]
- Cho, M.H.; Shim, S.M.; Lee, S.R.; Mar, W.; Kim, G.H. Effect of Evodiae fructus extracts on gene expressions related with alcohol metabolism and antioxidation in ethanol-loaded mice. Food Chem. Toxicol. 2005, 43, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- D’Souza El-Guindy, N.B.; Kovacs, E.J.; De Witte, P.; Spies, C.; Littleton, J.M.; De Villiers, W.J.; Lott, A.J.; Plackett, T.P.; Lanzke, N.; Meadows, G.G. Laboratory models available to study alcohol-induced organ damage and immune variations: Choosing the appropriate model. Alcohol. Clin. Exp. Res. 2010, 34, 1489–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, H.J.; Won, Y.S.; Park, O.; Chang, B.; Duryee, M.J.; Thiele, G.E.; Matsumoto, A.; Singh, S.; Abdelmegeed, M.A.; Song, B.J. Aldehyde dehydrogenase 2 deficiency ameliorates alcoholic fatty liver but worsens liver inflammation and fibrosis in mice. Hepatology 2014, 60, 146–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Zhu, P.; Jiang, C.; Ma, L.; Zhang, Z.; Zeng, X. Preliminary characterization, antioxidant activity in vitro and hepatoprotective effect on acute alcohol-induced liver injury in mice of polysaccharides from the peduncles of Hovenia dulcis. Food Chem. Toxicol. 2012, 50, 2964–2970. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-A.; Jung, Y.-S.; Lee, S.-J.; Park, S.-C.; Kim, M.-J.; Lee, E.-J.; Byun, H.-J.; Jhee, K.-H.; Lee, S.-P. Hepatoprotective effects of fermented field water-dropwort (Oenanthe javanica) extract and its major constituents. Food Chem. Toxicol. 2014, 67, 154–160. [Google Scholar] [CrossRef]
- OECD/OCDE. OECD Guidelines for the Testing of Chemicals, Section 4 Test No. 423: Acute Oral Toxicity-Acute Toxic Class Method. 2001. Available online: https://www.oecd-ilibrary.org/environment/test-no-423-acute-oral-toxicity-acute-toxic-class-method_9789264071001-en (accessed on 5 September 2016).
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health 2006, 29, 245–254. [Google Scholar] [PubMed]
- Husain, K.; Scott, B.R.; Reddy, S.K.; Somani, S.M. Chronic ethanol and nicotine interaction on rat tissue antioxidant defense system. Alcohol 2001, 25, 89–97. [Google Scholar] [CrossRef]
- Stoeckman, A.K.; Towle, H.C. The role of SREBP-1c in nutritional regulation of lipogenic enzyme gene expression. J. Biol. Chem. 2002, 277, 27029–27035. [Google Scholar] [CrossRef] [Green Version]
- Recknagel, R.O.; Glende, E.; Britton, R.S. Free radical damage and lipid peroxidation. Hepatotoxicology 1991, 401, 436. [Google Scholar]
- Donohue, T.M., Jr. Alcohol-induced steatosis in liver cells. World J. Gastroenterol. 2007, 13, 4974. [Google Scholar]
- Osborne, T.F. Sterol regulatory element-binding proteins (SREBPs): Key regulators of nutritional homeostasis and insulin action. J. Biol. Chem. 2000, 275, 32379–32382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoyama, T.; Peters, J.M.; Iritani, N.; Nakajima, T.; Furihata, K.; Hashimoto, T.; Gonzalez, F.J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPAR-alpha). J. Biol. Chem. 1998, 273, 5678–5684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quah, Y.; Lee, S.-J.; Lee, E.-B.; Birhanu, B.T.; Ali, M.; Abbas, M.A.; Boby, N.; Im, Z.-E.; Park, S.-C. Cornus officinalis Ethanolic Extract with Potential Anti-Allergic, Anti-Inflammatory, and Antioxidant Activities. Nutrients 2020, 12, 3317. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Hu, D.; Hou, L.B.; Song, L.Y.; Zhang, Y.J.; Xie, Y.; Tian, L.W. Phenolic Compounds from the Rhizomes of Smilax China L. and Their Anti-Inflammatory Activity. Molecules 2017, 22, 515. [Google Scholar] [CrossRef] [Green Version]
- SHIM, S.M. Changes in profiling of phenolic compounds, antioxidative effect and total phenolic content in Smilax China under in vitro physiological condition. J. Food Biochem. 2012, 36, 748–755. [Google Scholar] [CrossRef]
- Yoon, S.R.; Yang, S.H.; Suh, J.W.; Shim, S.M. Fermentation of Smilax China root by Aspergillus usami and Saccharomyces cerevisiae promoted concentration of resveratrol and oxyresveratrol and the free-radical scavenging activity. J. Sci. Food Agric. 2014, 94, 1822–1826. [Google Scholar] [CrossRef]
- Shao, B.; Guo, H.; Cui, Y.; Ye, M.; Han, J.; Guo, D. Steroidal saponins from Smilax China and their anti-inflammatory activities. Phytochemistry 2007, 68, 623–630. [Google Scholar] [CrossRef]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef] [Green Version]






| Treatment (per kg b.w.) | ALP (U/L) | ALT (U/L) | AST (U/L) | ALB (U/L) |
|---|---|---|---|---|
| ND | 857.0 ± 7.8 b | 107.3 ± 3.8 b | 26.3 ± 5.5 c | 4.0 ± 0.1 a |
| NC | 1364.7 ± 9.4 a | 169.3 ± 4.9 a | 47.3 ± 1.4 a | 4.0 ± 0.2 a |
| PC | 780.8 ± 4.8 b | 102.8 ± 2.1 b | 36.5 ± 4.7 b | 3.7 ± 0.1 a |
| FSC 125 | 1311.3 ± 4.1 a | 99.0 ± 9.4 b | 36.3 ± 4.4 b | 3.6 ± 0.2 a |
| FSC 250 | 1018.0 ± 7.4 c | 99.0 ± 6.1 b | 37.3 ± 4.4 ab | 3.5 ± 0.1 a |
| FSC 500 | 985.0 ± 9.7 b,c | 100.5 ± 9.6 b | 28.7 ± 1.2 c | 3.9 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boby, N.; Lee, E.-B.; Abbas, M.A.; Park, N.-H.; Lee, S.-P.; Ali, M.S.; Lee, S.-J.; Park, S.-C. Ethanol-Induced Hepatotoxicity and Alcohol Metabolism Regulation by GABA-Enriched Fermented Smilax china Root Extract in Rats. Foods 2021, 10, 2381. https://doi.org/10.3390/foods10102381
Boby N, Lee E-B, Abbas MA, Park N-H, Lee S-P, Ali MS, Lee S-J, Park S-C. Ethanol-Induced Hepatotoxicity and Alcohol Metabolism Regulation by GABA-Enriched Fermented Smilax china Root Extract in Rats. Foods. 2021; 10(10):2381. https://doi.org/10.3390/foods10102381
Chicago/Turabian StyleBoby, Naila, Eon-Bee Lee, Muhammad Aleem Abbas, Na-Hye Park, Sam-Pin Lee, Md. Sekendar Ali, Seung-Jin Lee, and Seung-Chun Park. 2021. "Ethanol-Induced Hepatotoxicity and Alcohol Metabolism Regulation by GABA-Enriched Fermented Smilax china Root Extract in Rats" Foods 10, no. 10: 2381. https://doi.org/10.3390/foods10102381