Plant Kunitz Inhibitors and Their Interaction with Proteases: Current and Potential Pharmacological Targets
Abstract
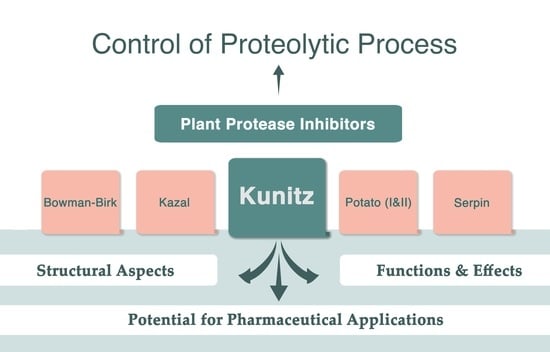
:1. Introduction
2. Plant Kunitz Inhibitors
3. Recombinant Kunitz Inhibitors
4. Defense Proteins
5. Antibacterial and Antifungal Activities
6. Inflammation
7. Coagulation and Thrombosis
8. Cancer
9. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richardson, M. Seed storage proteins: The Enzyme Inhibitors. In Methods in Plant Biochemistry Seed Proteins; Springer: Dordrecht, The Netherlands, 1991; Volume 5, pp. 259–305. [Google Scholar]
- Ussuf, K.K.; Laxmi, N.H.; Mitra, R. Proteinase inhibitors: Plant-derived genes of insecticidal protein for developing insect-resistant transgenic plants. Curr. Sci. 2001, 80, 847–853. Available online: http://www.jstor.org/stable/2410573570 (accessed on 22 March 2020).
- Srikanth, S.; Chen, Z. Plant Protease Inhibitors in Therapeutics-Focus on Cancer Therapy. Front. Pharmacol. 2016, 7, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergnolle, N. Protease inhibition as new therapeutic strategy for GI diseases. Gut 2016, 65, 1215–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- Bendre, A.D.; Ramasamy, S.; Suresh, C.G. Analysis of Kunitz inhibitors from plants for comprehensive structural and functional insights. Int. J. Biol. Macromol. 2018, 113, 933–943. [Google Scholar] [CrossRef]
- Benbow, H.R.; Jermiin, L.S.; Dohan, F.M. Serpins: Genome-Wide Characterisation and Expression Analysis of the Serine Protease Inhibitor Family in Triticum aestivum. G3 Genes|Genomes|Genet. 2019, 9, 2709–2722. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Ravet, K.; Pearce, S. Extensive structural variation in the Bowman-Birk inhibitor family in common wheat (Triticum aestivum L.). BMC Genom. 2021, 22, 218. [Google Scholar] [CrossRef]
- Pariani, S.; Contreras, M.; Rossi, F.R.; Sander, V.; Corigliano, M.G.; Simón, F.; Busi, M.V.; Gomez-Casati, D.F.; Pieckenstain, F.L.; Duschak, V.G.; et al. Characterization of a novel Kazal-type serine proteinase inhibitor of Arabidopsis thaliana. Biochimie 2016, 123, 85–94. [Google Scholar] [CrossRef]
- Oliva, M.L.V.; Silva, M.C.; Sallai, R.C.; Brito, M.V.; Sampaio, M.U. A novel subclassification for Kunitz proteinase inhibitors from leguminous seeds. Biochimie 2010, 92, 1667–1673. [Google Scholar] [CrossRef]
- Zhou, D.; Hansen, D.; Shabalin, I.G.; Gustchina, A.; Vieira, D.F.; De Brito, M.V.; Araujo, A.P.U.; Oliva, M.L.; Wlodawer, A. Structure of BbKI, a disulfide-free plasma kallikrein inhibitor. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71 Pt 8, 1055–1062. [Google Scholar] [CrossRef] [Green Version]
- Kunitz, M. Crystallization of a Trypsin Inhibitor from Soybean. Science 1945, 101, 668–669. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Macedo-Ribeiro, S.; Veríssimo, P.; Im, S.Y.; Sampaio, M.U.; Oliva, M.L.V. Crystal structure of a novel cysteinless plant Kunitz-type protease inhibitor. Biochem. Biophys. Res. Commun. 2007, 360, 735–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahata, A.M.; Bueno, N.R.; Rocha, H.A.; Franco, C.R.; Chammas, R.; Nakaie, C.R.; Jasiulionis, M.; Nader, H.B.; Santana, L.A.; Sampaio, M.U.; et al. Structural and inhibitory properties of a plant proteinase inhibitor containing the RGD motif. Int. J. Biol. Macromol. 2006, 40, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Srp, J.; Gustchina, A.; Dauter, Z.; Mares, M.; Wlodawer, A. Crystal structures of the complex of a kallikrein inhibitor from Bauhinia bauhinioides with trypsin and modeling of kallikrein complexes. Acta Crystallogr. Sect. D Struct. Biol. 2019, 75, 56–69. [Google Scholar] [CrossRef]
- Sumikawa, J.T.; Nakahata, A.M.; Fritz, H.; Mentele, R.; Sampaio, M.U.; Oliva, M.L. A Kunitz-Type Glycosylated Elastase Inhibitor with One Disulfide Bridge. Planta Med. 2006, 72, 393–397. [Google Scholar] [CrossRef]
- Cavalcanti, M.D.S.M.; Oliva, M.L.; Fritzc, H.; Jochumc, M.; Mentelec, R.; Sampaiob, M.; Coelho, L.C.; Batista, I.F.; Sampaio, C.A. Characterization of a Kunitz Trypsin Inhibitor with One Disulfide Bridge Purified from Swartzia pickellii. Biochem. Biophys. Res. Commun. 2002, 291, 635–639. [Google Scholar] [CrossRef]
- Lingaraju, M.H.; Gowda, L.R. A Kunitz trypsin inhibitor of Entada scandens seeds: Another member with single disulfide bridge. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2008, 1784, 850–855. [Google Scholar] [CrossRef]
- Macedo, M.L.; Garcia, V.A.; Freire, M.D.; Richardson, M. Characterization of a Kunitz trypsin inhibitor with a single disulfide bridge from seeds of Inga laurina (SW.) Willd. Phytochemistry 2007, 68, 1104–1111. [Google Scholar] [CrossRef]
- Krauchenco, S.; Nagem, R.A.; da Silva, J.A.; Marangoni, S.; Polikarpov, I. Three-dimensional structure of an unusual Kunitz (STI) type trypsin inhibitor from Copaifera langsdorffii. Biochimie 2004, 86, 167–172. [Google Scholar] [CrossRef]
- Song, H.K.; Suh, S.W. Kunitz-type soybean trypsin inhibitor revisited: Refined structure of its complex with porcine trypsin reveals an insight into the interaction between a homologous inhibitor from Erythrina caffra and tissue-type plasminogen activator. J. Mol. Biol. 1998, 275, 347–363. [Google Scholar] [CrossRef] [Green Version]
- Di Ciero, L.; Oliva, M.L.; Torquato, R.; Köhler, P.; Weder, J.K.; Novello, C.J.; Sampaio, C.A.; Oliveira, B.; Marangoni, S. The complete amino acid sequence of a trypsin inhibitor from Bauhinia variegata var. candida seeds. J. Protein Chem. 1998, 17, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Batista, I.F.; Nonato, M.C.; Bonfadini, M.R.; Beltramini, L.M.; Oliva, M.L.; Sampaio, M.U.; Sampaio, C.A.; Garratt, R.C. Preliminary crystallographic studies of EcTI, a serine proteinase inhibitor from Enterolobium contortisiliquum seeds. Acta Crystallogr. Sect. D Biol. Crystallogr. 2001, 57, 602–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, D.; Lobo, Y.; Batista, I.F.; Marques-Porto, R.; Gustchina, A.; Oliva, M.L.; Wlodawer, A. Crystal Structures of a Plant Trypsin Inhibitor from Enterolobium contortisiliquum (EcTI) and of Its Complex with Bovine Trypsin. PLoS ONE 2013, 8, e62252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patthy, A.; Molnár, T.; Porrogi, P.; Naude, R.; Gráf, L. Isolation and characterization of a protease inhibitor from Acacia karroo with a common combining loop and overlapping binding sites for chymotrypsin and trypsin. Arch. Biochem. Biophys. 2015, 565, 9–16. [Google Scholar] [CrossRef]
- Terada, S.; Katayama, H.; Noda, K.; Fujimura, S.; Kimoto, E. Amino Acid Sequences of Kunitz Family Subtilisin Inhibitors from Seeds of Canavalia lineata. J. Biochem. 1994, 115, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.P.; Hansen, D.; Vieira, D.F.; De Oliveira, C.; Santana, L.A.; Beltramini, L.M.; Sampaio, C.A.; Sampaio, M.U.; Oliva, M.L. Kunitz-type Bauhinia bauhinioides inhibitors devoid of disulfide bridges: Isolation of the cDNAs, heterologous expression and structural studies. Biol. Chem. 2005, 386, 561–568. [Google Scholar] [CrossRef]
- Sumikawa, J.T.; Brito, M.V.; Araújo, A.P.U.; Macedo, M.L.; Oliva, M.L.V.; Miranda, A. Action of Bauhinia-derivated compounds on Callosobruchus maculatus development. Adv. Exp. Med. Biol. 2009, 611, 563–564. [Google Scholar] [CrossRef]
- Ferreira, J.G.; Diniz, P.M.M.; de Paula, C.A.A.; Lobo, Y.A.; Gamero, E.J.P.; Paschoalin, T.; Nogueira-Pedro, A.; Maza, P.K.; Toledo, M.S.; Suzuki, E.; et al. The Impaired Viability of Prostate Cancer Cell Lines by the Recombinant Plant Kallikrein Inhibitor. J. Biol. Chem. 2013, 288, 13641–13654. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.-H.; Li, Y.-Y.; Xiang, M.; Zhu, J.-Q.; Huang, X.-H.; Wang, W.-J.; Tan, R.; Zhou, J.-Y.; Liao, H. Molecular cloning and characterization of α-amylase/subtilisin inhibitor from rhizome of Ligusticum chuanxiong. Biotechnol. Lett. 2016, 39, 141–148. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Q.; Li, J.; Zhang, G.; Jiamahate, A.; Zhou, J.; Liao, H. Isolation, structure modeling and function characterization of a trypsin inhibitor from Cassia obtusifolia. Biotechnol. Lett. 2015, 37, 863–869. [Google Scholar] [CrossRef]
- Cruz-Silva, I.; Gozzo, A.J.; Nunes, V.A.; Tanaka, A.S.; Araujo, M.D.S. Bioengineering of an elastase inhibitor from Caesalpinia echinata (Brazil wood) seeds. Phytochemistry 2021, 182, 112595. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yonekura, M.; Kouzuma, Y. Purification, cDNA cloning and characterization of Kunitz-type protease inhibitors from Apios americana tubers. Biosci. Biotechnol. Biochem. 2020, 84, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Bunyatang, O.; Chirapongsatonkul, N.; Bangrak, P.; Henry, R.; Churngchow, N. Molecular cloning and characterization of a novel bi-functional α-amylase/subtilisin inhibitor from Hevea brasiliensis. Plant Physiol. Biochem. 2016, 101, 76–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lacerda, J.T.J.G.; Lacerda, R.R.E.; Assunção, N.A.; Tashima, A.K.; Juliano, M.A.; dos Santos, G.A. New insights into lectin from Abelmoschus esculentus seeds as a Kunitz-type inhibitor and its toxic effects on Ceratitis capitata and root-knot nematodes Meloidogyne spp. Process Biochem. 2017, 63, 96–104. [Google Scholar] [CrossRef]
- De Oliveira, C.; Flores, T.M.D.O.; Cardoso, M.H.; Oshiro, K.G.N.; Russi, R.; de França, A.; Dos Santos, E.A.; Franco, O.L.; De Oliveira, A.S.; Migliolo, L. Dual Insecticidal Effects of Adenanthera pavonina Kunitz-Type Inhibitor on Plodia interpunctella is Mediated by Digestive Enzymes Inhibition and Chitin-Binding Properties. Molecules 2019, 24, 4344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meriño-Cabrera, Y.; Mendes, T.A.D.O.; Castro, J.; Barbosa, S.L.; Macedo, M.; Oliveira, M.G.A. Noncompetitive tight-binding inhibition of Anticarsia gemmatalis trypsins by Adenanthera pavonina protease inhibitor affects larvae survival. Arch. Insect Biochem. Physiol. 2020, 104, e21687. [Google Scholar] [CrossRef] [PubMed]
- Vajravijayan, S.; Pletnev, S.; Pletnev, V.Z.; Nandhagopal, N.; Gunasekaran, K. Crystal structure of a novel Kunitz type inhibitor, alocasin with anti-Aedes aegypti activity targeting midgut proteases. Pest Manag. Sci. 2018, 74, 2761–2772. [Google Scholar] [CrossRef] [PubMed]
- Valois, M.V.; de Oliveira, C.; Lapa, A.J.; Souccar, C.; Oliva, M.L.V. Bauhinia Protease Inhibitors Attenuate Gastric Ulcer by Blocking Neutrophil Enzymes. Planta Med. 2020, 87, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Bah, C.S.F.; Wong, J.H.; Pan, W.L.; Chan, Y.S.; Ye, X.J.; Ng, T.B. A potential human hepatocellular carcinoma inhibitor from Bauhinia purpurea L. seeds: From purification to mechanism exploration. Arch. Toxicol. 2011, 86, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Shamsi, T.N.; Parveen, R.; Ahamad, S. Structural and biophysical characterization of Cajanus cajan protease inhibitor. J. Nat. Sci. Biol. Med. 2017, 8, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandão-Costa, R.; Araújo, V.F.; Porto, A. CgTI, a novel thermostable Kunitz trypsin-inhibitor purified from Cassia grandis seeds: Purification, characterization and termiticidal activity. Int. J. Biol. Macromol. 2018, 118 Pt B, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.P.; Oliveira, J.T.; Rocha-Bezerra, L.C.; Sousa, D.O.; da Costa, H.P.S.; Araujo, N.M.; Carvalho, A.F.; Tabosa, P.M.; Monteiro-Moreira, A.C.; Lobo, M.D.; et al. A trypsin inhibitor purified from Cassia leiandra seeds has insecticidal activity against Aedes aegypti. Process Biochem. 2017, 57, 228–238. [Google Scholar] [CrossRef]
- Karray, A.; Alonazi, M.; Smaoui, S.; Michaud, P.; Soliman, D.; Ben Bacha, A. Purification and Biochemical Characterization of a New Protease Inhibitor from Conyza dioscoridis with Antimicrobial, Antifungal and Cytotoxic Effects. Molecules 2020, 25, 5452. [Google Scholar] [CrossRef] [PubMed]
- Oliva, L.V.; Almeida-Reis, R.; Theodoro-Junior, O.; Oliveira, B.M.; Leick, E.A.; Prado, C.M.; Brito, M.V.; Correia, M.T.D.S.; Paiva, P.M.; Martins, M.A.; et al. A plant proteinase inhibitor from Crataeva tapia (CrataBL) attenuates elastase-induced pulmonary inflammatory, remodeling, and mechanical alterations in mice. Process Biochem. 2015, 50, 1958–1965. [Google Scholar] [CrossRef] [Green Version]
- Bortolozzo, A.S.S.; Rodrigues, A.P.D.; Arantes-Costa, F.M.; Saraiva-Romanholo, B.M.; De Souza, F.C.R.; Brüggemann, T.R.; De Brito, M.V.; Ferreira, R.D.S.; Correia, M.T.D.S.; Paiva, P.M.G.; et al. The Plant Proteinase Inhibitor CrataBL Plays a Role in Controlling Asthma Response in Mice. BioMed Res. Int. 2018, 2018, 9274817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salu, B.R.; Ferreira, R.S.; Brito, M.V.; Ottaiano, T.F.; Cruz, J.W.M.; Silva, M.C.C.; Correia, M.T.S.; Paiva, P.M.; Maffei, F.H.A.; Oliva, M.L.V. CrataBL, a lectin and Factor Xa inhibitor, plays a role in blood coagulation and impairs thrombus formation. Biol. Chem. 2014, 395, 1027–1035. [Google Scholar] [CrossRef]
- Bonturi, C.R.; Silva, M.C.C.; Motaln, H.; Salu, B.R.; Ferreira, R.D.S.; Batista, F.P.; Correia, M.T.D.S.; Paiva, P.M.G.; Turnšek, T.L.; Oliva, M.L.V. A Bifunctional Molecule with Lectin and Protease Inhibitor Activities Isolated from Crataeva tapia Bark Significantly Affects Cocultures of Mesenchymal Stem Cells and Glioblastoma Cells. Molecules 2019, 24, 2109. [Google Scholar] [CrossRef] [Green Version]
- Salu, B.R.; Pando, S.C.; De Brito, M.V.; Medina, A.F.; Odei-Addo, F.; Frost, C.; Naude, R.; Sampaio, M.U.; Emsley, J.; Maffei, F.H.A.; et al. Improving the understanding of plasma kallikrein contribution to arterial thrombus formation using two plant protease inhibitors. Platelets 2018, 30, 305–313. [Google Scholar] [CrossRef]
- Ferreira, R.; Napoleão, T.H.; Silva-Lucca, R.A.; Silva, M.; Paiva, P.M.G.; Oliva, M.L.V. The effects of Enterolobium contortisiliquum serine protease inhibitor on the survival of the termite Nasutitermes corniger, and its use as affinity adsorbent to purify termite proteases. Pest Manag. Sci. 2019, 75, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, A.; Mayer, B.; Neth, P.; Hansen, D.; Sampaio, M.; Oliva, M. Blocking the Proliferation of Human Tumor Cell Lines by Peptidase Inhibitors from Bauhinia Seeds. Planta Med. 2013, 79, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paula, C.A.A.; Coulson-Thomas, V.J.; Ferreira, J.G.; Maza, P.K.; Suzuki, E.; Nakahata, A.M.; Nader, H.B.; Sampaio, M.U.; Oliva, M.L.V. Enterolobium contortisiliquum Trypsin Inhibitor (EcTI), a Plant Proteinase Inhibitor, Decreases in Vitro Cell Adhesion and Invasion by Inhibition of Src Protein-Focal Adhesion Kinase (FAK) Signaling Pathways. J. Biol. Chem. 2012, 287, 170–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonturi, C.R.; Motaln, H.; Silva, M.C.C.; Salu, B.R.; De Brito, M.V.; Costa, L.D.A.L.; Torquato, H.F.V.; Nunes, N.N.D.S.; Paredes-Gamero, E.J.; Turnšek, T.L.; et al. Could a plant derived protein potentiate the anticancer effects of a stem cell in brain cancer? Oncotarget 2018, 9, 21296–21312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, Y.A.; Bonazza, C.; Batista, F.P.; Castro, R.A.; Bonturi, C.R.; Salu, B.R.; Sinigaglia, R.D.C.; Toma, L.; Vicente, C.M.; Pidde, G.; et al. EcTI impairs survival and proliferation pathways in triple-negative breast cancer by modulating cell-glycosaminoglycans and inflammatory cytokines. Cancer Lett. 2020, 491, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Im, S.Y.; Bonturi, C.R.; Nakahata, A.M.; Nakaie, C.R.; Pott, A.; Pott, V.; Oliva, M.V. Differences in the Inhibitory Specificity Distinguish the Efficacy of Plant Protease Inhibitors on Mouse Fibrosarcoma. Plants 2021, 10, 602. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.F.; Oliveira, C.T.; Taveira, G.B.; Mello, E.D.O.; Gomes, V.M.; Macedo, M.L.R. Characterization of a Kunitz trypsin inhibitor from Enterolobium timbouva with activity against Candida species. Int. J. Biol. Macromol. 2018, 119, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Imran, M.; Ali, A.; Munawar, A.; Khaliq, B.; Anwar, F.; Saaed, Q.; Buck, F.; Hussain, S.; Saeed, A.; et al. Model prediction of a Kunitz-type trypsin inhibitor protein from seeds of Acacia nilotica L. with strong antimicrobial and insecticidal activity. Turk. J. Biol. 2020, 44, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Dib, H.X.; De Oliveira, D.G.L.; De Oliveira, C.F.R.; Taveira, G.B.; Mello, E.D.O.; Verbisck, N.V.; Chang, M.; Corrêa, D., Jr.; Gomes, V.M.; Macedo, M.L.R. Biochemical characterization of a Kunitz inhibitor from Inga edulis seeds with antifungal activity against Candida spp. Arch. Microbiol. 2018, 201, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Machado, S.W.; De Oliveira, C.; Zério, N.G.; Parra, J.; Macedo, M. Inga laurina trypsin inhibitor (ILTI) obstructs Spodoptera frugiperda trypsins expressed during adaptive mechanisms against plant protease inhibitors. Arch. Insect Biochem. Physiol. 2017, 95, e21393. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.S.; de Oliveira, C.F.R.; Machado, O.L.T.; de Mello, G.S.V.; Pitta, M.G.R.; Rêgo, M.J.B.M.; Napoleão, T.H.; Paiva, P.M.G.; Ribeiro, S.D.F.F.; Gomes, V.M.; et al. Exploiting the biological roles of the trypsin inhibitor from Inga vera seeds: A multifunctional Kunitz inhibitor. Process Biochem. 2016, 51, 792–803. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.-J.; Zhang, X.-P.; Xiao, R.; Li, P.-G. Sporamin suppresses growth of xenografted colorectal carcinoma in athymic BALB/c mice by inhibiting liver β-catenin and vascular endothelial growth factor expression. World J. Gastroenterol. 2019, 25, 3196–3206. [Google Scholar] [CrossRef]
- Roy, U.K.; Lavignac, N.; Rahman, A.M.; Nielsen, B.V. Purification of lectin and Kunitz trypsin inhibitor from soya seeds. J. Chromatogr. Sci. 2018, 56, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Xie, X.; Fu, N.; Wang, S. Physico-Chemical and Antifungal Properties of a Trypsin Inhibitor from the Roots of Pseudostellaria heterophylla. Molecules 2018, 23, 2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, R.S.; Brito, M.V.; Napoleão, T.H.; Silva, M.; Paiva, P.; Oliva, M. Effects of two protease inhibitors from Bauhinia bauhinoides with different specificity towards gut enzymes of Nasutitermes corniger and its survival. Chemosphere 2019, 222, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.V.; de Oliveira, C.; Salu, B.R.; Andrade, S.A.; Malloy, P.M.; Sato, A.C.; Vicente, C.P.; Sampaio, M.U.; Maffei, F.H.; Oliva, M.L.V. The Kallikrein Inhibitor from Bauhinia bauhinioides (BbKI) shows antithrombotic properties in venous and arterial thrombosis models. Thromb. Res. 2014, 133, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, S.S.; Gujjarlapudi, M.; Lokya, V.; Mallikarjuna, N.; Dutta-Gupta, A.; Padmasree, K. Purification and characterization of Bowman-Birk and Kunitz isoinhibitors from the seeds of Rhynchosia sublobata (Schumach.) Meikle, a wild relative of pigeonpea. Phytochemistry 2019, 159, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; He, Y.; Yan, X.; Liu, L.; Guo, R.; Xia, X.; Cheng, D.; Mi, X.; Samarina, L.; Liu, S.; et al. Duplication and transcriptional divergence of three Kunitz protease inhibitor genes that modulate insect and pathogen defenses in tea plant (Camellia sinensis). Hortic. Res. 2019, 6, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendonça, E.G.; Barros, R.A.; Cordeiro, G.; Silva, C.R.; Campos, W.G.; Oliveira, J.A.; Oliveira, M.G.A. Larval development and proteolytic activity of Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae) exposed to different soybean protease inhibitors. Arch. Insect Biochem. Physiol. 2020, 103, e21637. [Google Scholar] [CrossRef]
- Sasaki, D.Y.; Jacobowski, A.C.; de Souza, A.P.; Cardoso, M.H.; Franco, O.L.; Macedo, M.L.R. Effects of proteinase inhibitor from Adenanthera pavonina seeds on short- and long term larval development of Aedes aegypti. Biochimie 2015, 112, 172–186. [Google Scholar] [CrossRef]
- Swathi, M.; Mishra, P.K.; Lokya, V.; Swaroop, V.; Mallikarjuna, N.; Dutta-Gupta, A.; Padmasree, K. Purification and Partial Characterization of Trypsin-Specific Proteinase Inhibitors from Pigeonpea Wild Relative Cajanus platycarpus L. (Fabaceae) Active against Gut Proteases of Lepidopteran Pest Helicoverpa armigera. Front. Physiol. 2016, 7, 388. [Google Scholar] [CrossRef] [Green Version]
- Orona-Tamayo, D.; Wielsch, N.; Blanco-Labra, A.; Svatos, A.; Farías-Rodríguez, R.; Heil, M. Exclusive rewards in mutualisms: Ant proteases and plant protease inhibitors create a lock-key system to protect Acacia food bodies from exploitation. Mol. Ecol. 2013, 22, 4087–4100. [Google Scholar] [CrossRef]
- Sanon, A.; Zakaria, I.; Clémentine, L.D.B.; Niango, B.M.; Honora, N.R.C. Potential of Botanicals to Control Callosobruchus maculatus (Col.: Chrysomelidae, Bruchinae), a Major Pest of Stored Cowpeas in Burkina Faso: A Review. Int. J. Insect. Sci. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallqui, K.S.V.; Oliveira, E.E.; Guedes, R.N.C. Competition between the bean weevils Acanthoscelides obtectus and Zabrotes subfasciatus in common beans. J. Stored Prod. Res. 2013, 55, 32–35. [Google Scholar] [CrossRef]
- Rufino, F.P.; Pedroso, V.M.; Araujo, J.N.; de França, A.F.; Rabelo, L.M.; Migliolo, L.; Kiyota, S.; Santos, E.A.; Franco, O.L.; Oliveira, A.S. Inhibitory effects of a Kunitz-type inhibitor from Pithecellobium dumosum (Benth) seeds against insect-pests’ digestive proteinases. Plant Physiol. Biochem. 2013, 63, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.; Schlüter, O. Insect-based protein sources and their potential for human consumption: Nutritional composition and processing. Anim. Front. 2015, 5, 20–24. [Google Scholar] [CrossRef]
- Sallai, R.C.; Salu, B.R.; Silva-Lucca, R.A.; Alves, F.L.; Napoleão, T.H.; Paiva, P.M.G.; Ferreira, R.D.S.; Sampaio, M.U.; Oliva, M.L.V. Biotechnological Potential of Araucaria angustifolia Pine Nuts Extract and the Cysteine Protease Inhibitor AaCI-2S. Plants 2020, 9, 1676. [Google Scholar] [CrossRef]
- Müller, V.; Bonacci, G.; Batthyány, C.; Amé, M.V.; Carrari, F.; Gieco, J.; Asis, R. Peanut Seed Cultivars with Contrasting Resistance to Aspergillus parasiticus Colonization Display Differential Temporal Response of Protease Inhibitors. Phytopathology 2017, 107, 474–482. [Google Scholar] [CrossRef]
- Tripathi, V.R.; Kumar, S.; Garg, S.K. A study on trypsin, Aspergillus flavus and Bacillus sp. protease inhibitory activity in Cassia tora (L.) syn Senna tora (L.) Roxb. seed extract. BMC Complement. Altern. Med. 2011, 11, 56. [Google Scholar] [CrossRef] [Green Version]
- Barros, K.M.A.; Sardi, J.D.C.O.; Maria-Neto, S.; Macedo, A.J.; Ramalho, S.R.; de Oliveira, D.G.L.; Macedo, M.L.R. A new Kunitz trypsin inhibitor from Erythrina poeppigiana exhibits antimicrobial and antibiofilm properties against bacteria. Biomed. Pharmacother. 2021, 144, 112198. [Google Scholar] [CrossRef]
- Araújo, N.M.; Dias, L.P.; Costa, H.P.; Sousa, D.O.; Vasconcelos, I.M.; de Morais, G.A.; Oliveira, J.T. ClTI, a Kunitz trypsin inhibitor purified from Cassia leiandra Benth. seeds, exerts a candidicidal effect on Candida albicans by inducing oxidative stress and necrosis. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 183032. [Google Scholar] [CrossRef]
- Georgiadou, A.; Lee, H.J.; Walther, M.; van Beek, A.; Fitriani, F.; Wouters, D.; Kuijpers, T.W.; Nwakanma, D.; D’Alessandro, U.; Riley, E.M.; et al. Modelling pathogen load dynamics to elucidate mechanistic determinants of host–Plasmodium falciparum interactions. Nat. Microbiol. 2019, 4, 1592–1602. [Google Scholar] [CrossRef]
- Morais, S.B.; Figueiredo, B.C.; Assis, N.R.G.; Alvarenga, D.M.; De Magalhães, M.T.Q.; Ferreira, R.S.; Vieira, A.T.; Menezes, G.B.; Oliveira, S.C. Schistosoma mansoni SmKI-1 serine protease inhibitor binds to elastase and impairs neutrophil function and inflammation. PLoS Pathog. 2018, 14, e1006870. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, S.; Unger, B.L.; Comstock, A.T.; Angel, K.A.; Mancuso, P.; Martinez, F.J.; Sajjan, U.S. Aberrantly activated EGFR contributes to enhanced IL-8 expression in COPD airways epithelial cells via regulation of nuclear FoxO3A. Thorax 2012, 68, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins-Olivera, B.T.; Almeida-Reis, R.; Theodoro-Júnior, O.A.; Oliva, L.V.; Nunes, N.N.D.S.; Olivo, C.R.; de Brito, M.V.; Prado, C.M.; Leick, E.A.; Martins, M.D.A.; et al. The Plant-Derived Bauhinia bauhinioides Kallikrein Proteinase Inhibitor (rBbKI) Attenuates Elastase-Induced Emphysema in Mice. Mediat. Inflamm. 2016, 2016, 5346574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida-Reis, R.; Theodoro-Junior, O.A.; Oliveira, B.T.M.; Oliva, L.V.; Toledo-Arruda, A.; Bonturi, C.R.; Brito, M.V.; Lopes, F.D.T.Q.S.; Prado, C.M.; Florencio, A.C.; et al. Plant Proteinase Inhibitor BbCI Modulates Lung Inflammatory Responses and Mechanic and Remodeling Alterations Induced by Elastase in Mice. BioMed Res. Int. 2017, 2017, 8287125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.; Bortolozzo, A.; Arantes-Costa, F.M.; Saraiva-Romanholo, B.M.; de Souza, F.; Brüggemann, T.R.; Santana, F.; de Brito, M.V.; Bonturi, C.R.; Nunes, N.; et al. A plant proteinase inhibitor from Enterolobium contortisiliquum attenuates airway hyperresponsiveness, inflammation and remodeling in a mouse model of asthma. Histol. Histopathol. 2019, 34, 537–552. [Google Scholar] [CrossRef]
- Rakashanda, S.; Qazi, A.K.; Majeed, R.; Rafiq, S.; Dar, I.M.; Masood, A.; Hamid, A.; Amin, S. Antiproliferative activity of Lavatera cashmeriana- protease inhibitors towards human cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 3975–3978. [Google Scholar] [CrossRef] [Green Version]
- Schmaier, A.H. Antithrombotic potential of the contact activation pathway. Curr. Opin. Hematol. 2016, 23, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Kolte, D.; Shariat-Madar, Z. Plasma Kallikrein Inhibitors in Cardiovascular Disease: An Innovative Therapeutic Approach. Cardiol. Rev. 2016, 24, 99–109. [Google Scholar] [CrossRef]
- Simão, F.; Feener, E.P. The Effects of the Contact Activation System on Hemorrhage. Front. Med. 2017, 4, 121. [Google Scholar] [CrossRef] [Green Version]
- Ottaiano, T.F.; Andrade, S.S.; de Oliveira, C.; Silva, M.C.; Buri, M.V.; Juliano, M.A.; Girão, M.J.; Sampaio, M.U.; Schmaier, A.H.; Wlodawer, A.; et al. Plasma kallikrein enhances platelet aggregation response by subthreshold doses of ADP. Biochimie 2017, 135, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Geddings, J.E.; Mackman, N. Comment on “Tissue factor expressed by microparticles is associated with mortality but not with thrombosis in cancer patients”. Thromb. Haemost. 2014, 111, 180–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahata, A.M.; Mayer, B.; Ries, C.; de Paula, C.A.A.; Karow, M.; Neth, P.; Sampaio, M.U.; Jochum, M.; Oliva, M.L.V. The effects of a plant proteinase inhibitor from Enterolobium contortisiliquum on human tumor cell lines. Biol. Chem. 2011, 392, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Pathak, M.; Manna, R.; Li, C.; Kaira, B.G.; Hamad, B.K.; Belviso, B.D.; Bonturi, C.R.; Dreveny, I.; Fischer, P.M.; Dekker, L.V.; et al. Crystal structures of the recombinant β-factor XIIa protease with bound Thr-Arg and Pro-Arg substrate mimetics. Acta Crystallogr. Sect. D Struct. Biol. 2019, 75 Pt 6, 578–591. [Google Scholar] [CrossRef]
- Oliva, M.L.V.; Ferreira, R.D.S.; Ferreira, J.G.; De Paula, C.A.A.; Salas, C.E.; Sampaio, M.U. Structural and functional properties of kunitz proteinase inhibitors from leguminosae: A mini review. Curr. Protein Pept. Sci. 2011, 12, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Decrem, Y.; Rath, G.; Blasioli, V.; Cauchie, P.; Robert, S.; Beaufays, J.; Frère, J.-M.; Feron, O.; Dogné, J.-M.; Dessy, C.; et al. Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J. Exp. Med. 2009, 206, 2381–2395. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.; Valois, M.V.; Ottaiano, T.F.; Miranda, A.; Hansen, D.; Sampaio, M.U.; Oliva, M.L.V.; Maffei, F.H.D.A. The recombinant plant Bauhinia bauhinioides elastase inhibitor reduces rat thrombus without alterations in hemostatic parameters. Sci. Rep. 2021, 11, 13475. [Google Scholar] [CrossRef] [PubMed]
- Kearney, K.J.; Butler, J.; Posada, O.M.; Wilson, C.; Heal, S.; Ali, M.; Hardy, L.; Ahnström, J.; Gailani, D.; Foster, R.; et al. Kallikrein directly interacts with and activates Factor IX, resulting in thrombin generation and fibrin formation independent of Factor XI. Proc. Natl. Acad. Sci. USA 2021, 118, 218–239. [Google Scholar] [CrossRef]
- Eatemadi, A.; Aiyelabegan, H.T.; Negahdari, B.; Mazlomi, M.A.; Daraee, H.; Daraee, N.; Eatemadi, R.; Sadroddiny, E. Role of protease and protease inhibitors in cancer pathogenesis and treatment. Biomed. Pharmacother. 2017, 86, 221–231. [Google Scholar] [CrossRef]
- Wilkinson, R.; Woods, K.; D’Rozario, R.; Prue, R.; Vari, F.; Hardy, M.; Dong, Y.; Clements, J.; Hart, D.N.J.; Radford, K. Human kallikrein 4 signal peptide induces cytotoxic T cell responses in healthy donors and prostate cancer patients. Cancer Immunol. Immunother. 2011, 61, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Prassas, I.; Eissa, A.; Poda, G.; Diamandis, E.P. Unleashing the therapeutic potential of human kallikrein-related serine proteases. Nat. Rev. Drug Discov. 2015, 14, 183–202. [Google Scholar] [CrossRef]
- Ossovskaya, V.S.; Bunnett, N.W. Protease-Activated Receptors: Contribution to Physiology and Disease. Physiol. Rev. 2004, 84, 579–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obiezu, C.V.; Diamandis, E.P. Human tissue kallikrein gene family: Applications in cancer. Cancer Lett. 2005, 224, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Trabocchi, A.; Menchi, G.; Cini, N.; Bianchini, F.; Raspanti, S.; Bottoncetti, A.; Pupi, A.; Calorini, L.; Guarna, A. Click-Chemistry-Derived Triazole Ligands of Arginine−Glycine−Aspartate (RGD) Integrins with a Broad Capacity To Inhibit Adhesion of Melanoma Cells and Both in Vitro and in Vivo Angiogenesis. J. Med. Chem. 2010, 53, 7119–7128. [Google Scholar] [CrossRef] [PubMed]
- Tantivejkul, K.; Loberg, R.D.; Mawocha, S.C.; Day, L.L.; John, L.S.; Pienta, B.A.; Rubin, M.; Pienta, K. PAR1-mediated NFκB activation promotes survival of prostate cancer cells through a Bcl-xL-dependent mechanism. J. Cell. Biochem. 2005, 96, 641–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilgin, M.; Burgazli, K.M.; Rafiq, A.; Mericliler, M.; Neuhof, C.; Oliva, M.L.; Parahuleva, M.; Soydan, N.; Doerr, O.; Abdallah, Y.; et al. Effect of bauhinia bauhinioides kallikrein inhibitor on endothelial proliferation and intracellular calcium concentration. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 46–51. [Google Scholar] [PubMed]



| Structural Characteristics | Serine Proteases Inhibition (Ki nM) | Cysteine Proteases Inhibition (Ki nM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitor | Cys Residues/S-S bonds/ Chains | Digestion Enzymes | Coagulation & Fibrinolysis Enzymes | Inflammatory Enzymes | Inflammatory Enzymes | |||||||||
| Cys | S-S | Chains | Trypsin (Bovine) | Chymotrypsin (Bovine) | FXIIa | PKa (Human) | PKa (Rat) | FXa | Plasmin | HNE | Cat G | Cat L | Cat B | |
| EcTI | 4 | 2 | 2 | 0.9 | 1.1 | 82.0 | 55.0 | n.d. | n.i. | 9.4 | 55 | n.d. | n.d. | n.d. |
| BbKI | 1 | 0 | 1 | 0.6 | 2.7 | n.d. | 4.7 | 5.2 | n.i. | 33.0 | n.i. | n.i. | n.i. | n.i. |
| rBbKI | 1 | 0 | 1 | 20.0 | n.i. | n.d. | 2.0 | n.d. | n.d. | 33.0 | n.i. | n.i. | n.i. | n.i. |
| rBbKI-R64A | 1 | 0 | 1 | 25.0 | n.i. | n.d. | 98.0 | n.d. | n.d. | 2.6 | n.i. | n.d. | n.i. | n.d. |
| BrTI | 0 | 0 | 1 | 2.9 | n.i. | n.d. | 14 | 1.3 | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| BbCI | 0 | 0 | 1 | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 5.3 | 160.0 | 0.2 | n.i. |
| rBbCI | 0 | 0 | 1 | n.i. | n.i. | n.d. | n.i. | n.d. | n.d. | n.i. | 1.7 | 210.0 | 9.0 | n.i. |
| BuXI | 4 | 2 | 1 | 21.0 | 28.0 | 74.0 | 6.9 | n.d. | 14.0 | 76.0 | n.i. | n.i. | n.d. | n.d. |
| BvTI-C | 4 | 2 | 1 | 6.9 | n.d. | n.d. | 4.5 | n.d. | n.i. | n.d. | n.d. | n.d. | n.d. | n.d. |
| BvTI | 4 | 2 | 1 | 1.6 | 1.2 | 110 | 80.0 | 2.2 | n.i. | 2.9 | n.i. | n.d. | n.d. | n.d. |
| Plant Kunitz Inhibitor | Identification | Action |
|---|---|---|
| Abelmoschus esculentus lectin and Kunitz inhibitor | AEL | Toxicity against the fruit fly Ceratitis capitata and the nematodes Meloidogyne incognita and M. javanica [35] |
| Adenanthera pavonina Kunitz-type inhibitor | ApKTI | Insecticidal activity against Plodia interpunctella larvae [36] and reduces the survival of neonatal Anticarsia gemmatalis larvae [37] |
| Alocasia macrorrhiza Kunitz-type inhibitor | Alocasin | Increases the mortality rate of Aedes aegypti larvae and adult females [38] |
| Bauhinia bauhinioides cruzipain inhibitor and B. mollis elastase inhibitor | BbCI and BmEI | Anti-ulcer effects: regulation of pro-inflammatory processes and stress-induced gastric mucosal injuries [39] |
| Bauhinia purpurea L. trypsin inhibitor | BPLTI | Anti-proliferative and pro-apoptotic activities in HepG2, MCF-7, CNE-1, and CNE-2 cells [40] |
| Cajanus cajan L. Kunitz-type inhibitor | CCPI | Acts on the adenocarcinoma human alveolar basal epithelial cell line A549 [41] |
| Cassia grandis trypsin inhibitor | CGTI | Termiticidal activity against Nasutitermes corniger [42] |
| Cassia leiandra trypsin inhibitor | ClTI | Inhibits larvae development and delays adult emergence in A. aegypti causing mortality [43] |
| Conyza dioscoridis protease inhibitor | PDInhibitor | Cytotoxic effects on MDA-MB-231, HCT-116, and LoVo, without affecting HUVECs [44] |
| Crataeva tapia bark lectin | CrataBL | Controls the inflammatory response with a decrease in bronchoalveolar lavage, number of neutrophils, lymphocytes, eosinophils and mean alveolar diameter [45,46]; Impairs thrombus formation [47] and U87 cell microenvironment [48] |
| Delonix regia trypsin inhibitor and Acacia schweinfurthii trypsin inhibitor | DrTI and AsTI | Prolong the time for total carotid artery occlusion in mice and prevent arterial thrombus formation, without affecting bleeding time [49] |
| Enterolobium contortisiliquum trypsin inhibitor | EcTI | Induces the death of Nasutitermes corniger worker termites [50]; Cytotoxic to colorectal cancer cells (HCT116 and HT29), breast cancer cells (SkBr-3, MDA-MB-231 and MCF-7), human leukemia cell lines (K562 and THP-1), human gastric cancer (Hs746T), glioblastoma (U87) and mouse fibrosarcoma (L929), without affecting normal human primary fibroblasts and human mesenchymal stem cells (hMSCs) [51,52,53,54,55] |
| Enterolobium timbouva trypsin inhibitor | EtTI | Affects the integrity of the plasma membrane of yeasts, inducing the generation of intracellular ROS [56,57] |
| Recombinant Hevea brasiliensis amylase/subtilisin inhibitor | rHbASI | High activity against Aspergillus oryzae and inhibited mycelial growth of Phytophthora palmivora [34] |
| Inga edulis trypsin inhibitor | IeTI | Inhibits the pathogenic fungi Candida tropicalis and Candida buinensis [58] |
| Inga laurina trypsin inhibitor | ILTI | Inhibits trypsin midgut of lepidopteran pest: S. frugiperda [59] |
| Inga vera trypsin inhibitor | IVTI | Midgut inhibition of lepidopteran pests: S. frugiperda, C. cephalonica, H. virescens, and H. zea [60] |
| Ipomoea potatoes Kunitz-type trypsin inhibitor | Sporamin | Suppress the growth of colorectal tumor nodules in mice [61] |
| Kunitz-type trypsin inhibitor | KTI-A | Anti-proliferative activity in the melanoma cell line, B16F1 [62] |
| Pseudostellaria heterophylla trypsin inhibitor | PhTI | Potent activity against the pathogenic fungi Candida gloeosporioides and Fusarium oxysporum [63] |
| Recombinant B. bauhinioides kallikrein inhibitor | rBbKI | Termiticidal activity in workers termites (Nasutitermes corniger) [64]; Antithrombotic properties in venous and arterial thrombosis models [65]; Inhibits cell viability of MKN-28, HT-29, HCT 116, Hs746T [51] and L929 [55] |
| Recombinant B. bauhinioides kallikrein inhibitor modified | rBbKIm | Defense against Callosobruchus maculatus development [28]; Reduces cell viability in prostate cancer (DU145/PC3) leads to cell death [29] |
| Rhynchosia sublobata Kunitz isoinhibitor | RsKI | Actives against lepidopteran gut proteases: Achaea Janata and Helicoverpa armigera [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonturi, C.R.; Silva Teixeira, A.B.; Rocha, V.M.; Valente, P.F.; Oliveira, J.R.; Filho, C.M.B.; Fátima Correia Batista, I.; Oliva, M.L.V. Plant Kunitz Inhibitors and Their Interaction with Proteases: Current and Potential Pharmacological Targets. Int. J. Mol. Sci. 2022, 23, 4742. https://doi.org/10.3390/ijms23094742
Bonturi CR, Silva Teixeira AB, Rocha VM, Valente PF, Oliveira JR, Filho CMB, Fátima Correia Batista I, Oliva MLV. Plant Kunitz Inhibitors and Their Interaction with Proteases: Current and Potential Pharmacological Targets. International Journal of Molecular Sciences. 2022; 23(9):4742. https://doi.org/10.3390/ijms23094742
Chicago/Turabian StyleBonturi, Camila Ramalho, Ana Beatriz Silva Teixeira, Vitória Morais Rocha, Penélope Ferreira Valente, Juliana Rodrigues Oliveira, Clovis Macêdo Bezerra Filho, Isabel Fátima Correia Batista, and Maria Luiza Vilela Oliva. 2022. "Plant Kunitz Inhibitors and Their Interaction with Proteases: Current and Potential Pharmacological Targets" International Journal of Molecular Sciences 23, no. 9: 4742. https://doi.org/10.3390/ijms23094742