Conditions for Achieving Postoperative Pelvic Incidence-Lumbar Lordosis < 10° in Circumferential Minimally Invasive Surgery for Adult Spinal Deformity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
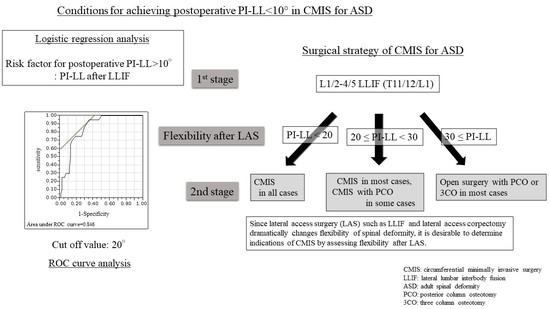
2.2. Surgical Method
2.3. Radiological Evaluation
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Radiographic Parameters
3.3. Clinical Outcomes
3.4. Complications
4. Discussion
4.1. Pros and Cons of Staged Surgery
4.2. Correction Force/Indications for CMIS for ASD
4.3. Complications
4.4. Proximal Junctional Kyphosis
4.5. Rod Fractures
4.6. Infection
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bess, S.; Protopsaltis, T.S.; Lafage, V.; Lafage, R.; Ames, C.P.; Errico, T.; Smith, J.S. Clinical and radiographic evaluation of adult spinal deformity. Clin. Spine Surg. 2016, 29, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Bess, S.; Line, B.; Fu, K.M.; McCarthy, I.; Lafage, V.; Schwab, F.; Shaffrey, C.; Ames, C.; Akbarnia, B.; Jo, H.; et al. The health impact of symptomatic adult spinal deformity: Comparison of deformity types to United States population norms and chronic diseases. Spine 2016, 41, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Acaroglu, E.; Yavuz, A.C.; Guler, U.O.; Yuksel, S.; Yavuz, Y.; Domingo-Sabat, M.; Pellise, F.; Alanay, A.; Perez Grueso, F.S.; Kleinstück, F.; et al. A decision analysis to identify the ideal treatment for adult spinal deformity: Is surgery better than non-surgical treatment in improving health-related quality of life and decreasing the disease burden? Eur. Spine J. 2016, 25, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Djurasovic, M.; Glassman, S.D. Correlation of radiographic and clinical findings in spinal deformities. Neurosurg. Clin. N. Am. 2007, 18, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Gum, J.L.; Glassman, S.D.; Douglas, L.R.; Carreon, L.Y. Correlation between cervical spine sagittal alignment and clinical outcome after anterior cervical discectomy and fusion. Am. J. Orthop. 2012, 41, E81–E84. [Google Scholar]
- Lenke, L.G.; Fehlings, M.G.; Shaffrey, C.I.; Cheung, K.M.; Carreon, L.; Dekutoski, M.B.; Schwab, F.J.; Boachie-Adjei, O.; Kebaish, K.M.; Ames, C.P.; et al. Neurologic outcomes of complex adult spinal deformity surgery: Results of the prospective, multicenter Scoli-RISK-1 Study. Spine 2016, 41, 204–212. [Google Scholar] [CrossRef]
- Schwab, F.; Patel, A.; Ungar, B.; Farcy, J.P.; Lafage, V. Adult spinal deformity-postoperative standing imbalance: Adult spinal deformity-postoperative standing imbalance: How much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine 2010, 35, 2224–2231. [Google Scholar] [CrossRef]
- Kothari, P.; Lee, N.J.; Leven, D.M.; Lakomkin, N.; Shin, J.I.; Skovrlj, B.; Steinberger, J.; Guzman, J.Z.; Cho, S.K. Impact of gender on 30-day complications after adult spinal deformity surgery. Spine 2016, 41, 1133–1138. [Google Scholar] [CrossRef] [Green Version]
- Maruo, K.; Ha, Y.; Inoue, S.; Samuel, S.; Okada, E.; Hu, S.S.; Deviren, V.; Burch, S.; William, S.; Ames, C.P.; et al. Predictive factors for proximal junctional kyphosis in long fusions to the sacrum in adult spinal deformity. Spine 2013, 38, E1469–E1476. [Google Scholar] [CrossRef]
- Mok, J.M.; Cloyd, J.M.; Bradford, D.S.; Hu, S.S.; Deviren, V.; Smith, J.A.; Tay, B.; Berven, S.H. Reoperation after primary fusion for adult spinal deformity: Rate, reason, and timing. Spine 2009, 34, 832–839. [Google Scholar] [CrossRef]
- Pichelmann, M.A.; Lenke, L.G.; Bridwell, K.H.; Good, C.R.; O’Leary, P.T.; Sides, B.A. Revision rates following primary adult spinal deformity surgery: Six hundred forty-three consecutive patients followed-up to twenty-two years postoperative. Spine 2010, 35, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, B.M.; Aryan, H.E.; Pimenta, L.; Taylor, W.R. Extreme lateral interbody fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006, 6, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Mummaneni, P.V.; Park, P.; Fu, K.M.; Wang, M.Y.; Nguyen, S.; Lafage, V.; Uribe, J.S.; Ziewacz, J.; Terran, J.; Okonkwo, D.O.; et al. Does minimally invasive percutaneous posterior instrumentation reduce risk of proximal junctional kyphosis in adult spinal deformity surgery? A propensity-matched cohort analysis. Neurosurgery 2016, 78, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.M.; Mundis, G.M., Jr.; Ahmed, Y.; El Ahmadieh, T.Y.; Wang, M.Y.; Mummaneni, P.V.; Uribe, J.S.; Okonkwo, D.O.; Eastlack, R.K.; Anand, N.; et al. Comparison of radiographic results after minimally invasive, hybrid, and open surgery for adult spinal deformity: A multicenter study of 184 patients. Neurosurg. Focus 2014, 36, E13. [Google Scholar] [CrossRef] [PubMed]
- Eastlack, R.K.; Srinivas, R.; Mundis, G.M.; Nguyen, S.; Mummaneni, P.V.; Okonkwo, D.O.; Kanter, A.S.; Anand, N.; Park, P.; Nunley, P.; et al. Early and late reoperation rates with various MIS techniques for adult spinal deformity correction. Glob. Spine J. 2019, 9, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheer, J.K.; Fakurnejad, S.; Lau, D.; Daubs, M.D.; Coe, J.D.; Paonessa, K.J.; LaGrone, M.O.; Amaral, R.A.; Trobisch, P.D.; Lee, J.-H.; et al. Results of the 2014 SRS survey on PJK/PJF: A report on variation of select SRS member practice patterns, treatment indications, and opinions on classification development. Spine 2015, 40, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Glassman, S.D.; Hamill, C.L.; Bridwell, K.H.; Schwab, F.J.; Dimar, J.R.; Lowe, T.G. The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine 2007, 32, 2764–2770. [Google Scholar] [CrossRef]
- Smith, J.S.; Klineberg, E.; Lafage, V.; Shaffrey, C.I.; Schwab, F.; Lafage, R.; Hostin, R.; Mundis, G.M.; Errico, T.J.; Kim, H.J.; et al. Prospective multicenter assessment of perioperative and minimum 2-year postoperative complication rates associated with adult spinal deformity surgery. J. Neurosurg. Spine 2016, 25, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Park, P.; Wang, M.Y.; Lafage, V.; Nguyen, S.; Ziewacz, J.; Okonkwo, D.O.; Uribe, J.S.; Eastlack, R.K.; Anand, N.; Haque, R.; et al. Comparison of two minimally invasive surgery strategies to treat adult spinal deformity. J. Neurosurg. Spine 2015, 22, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Than, K.D.; Park, P.; Tran, S.; Mundis, G.M.; Fu, K.M.; Uribe, J.S.; Okonkwo, D.O.; Nunley, P.D.; Fessler, R.G.; Eastlack, R.K.; et al. Analysis of complications with staged surgery for less invasive treatment of adult spinal deformity. World Neurosurg. 2019, 126, e1337–e1342. [Google Scholar] [CrossRef]
- Anand, N.; Kong, C.; Fessler, R.G. A staged protocol for circumferential minimally invasive surgical correction of adult spinal deformity. Neurosurgery 2017, 81, 733–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mummaneni, P.V.; Shaffrey, C.I.; Lenke, L.G.; Park, P.; Wang, M.Y.; La Marca, F.; Smith, J.S.; Mundis, G.M.; Okonkwo, D.O.; Moal, B.; et al. The minimally invasive spinal deformity surgery algorithm: A reproducible rational framework for decision making in minimally invasive spinal deformity surgery. Neurosurg. Focus 2014, 36, E6. [Google Scholar] [CrossRef] [PubMed]
- Mummaneni, P.V.; Park, P.; Shaffrey, C.I.; Wang, M.Y.; Uribe, J.S.; Fessler, R.G.; Chou, D.; Kanter, A.S.; Okonkwo, D.O.; Mundis, G.M.; et al. The MISDEF2 algorithm: An updated algorithm for patient selection in minimally invasive deformity surgery. J. Neurosurg. Spine 2019, 32, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Mundis, G.; Uribe, J.S.; Mummaneni, P.V.; Anand, N.; Park, P.; Okonkwo, D.O.; Kanter, A.S.; Fessler, R.G.; Nguyen, S.; Akbarnia, B.A.; et al. 172-A critical analysis of sagittal plane deformity correction with minimally invasive surgery: A 2-year follow-up study of deformity patients categorized by the SRS-Schwab Classification. Neurosurgery 2015, 62, 222–223. [Google Scholar] [CrossRef]
- Yamato, Y.; Hasegawa, T.; Kobayashi, S.; Yasuda, T.; Togawa, D.; Arima, H.; Oe, S.; Iida, T.; Matsumura, A.; Hosogane, N.; et al. Calculation of the target lumbar lordosis angle for restoring an optimal pelvic tilt in elderly patients with adult spinal deformity. Spine 2016, 41, E211–E217. [Google Scholar] [CrossRef]
- Hasegawa, K.; Okamoto, M.; Hatsushikano, S.; Shimoda, H.; Ono, M.; Watanabe, K. Normative values of spino-pelvic sagittal alignment, balance, age, and health-related quality of life in a cohort of healthy adult subjects. Eur. Spine J. 2016, 25, 3675–3686. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.Y.; Mummaneni, P.V.; Fu, K.M.; Anand, N.; Okonkwo, D.O.; Kanter, A.S.; La Marca, F.; Fessler, R.; Uribe, J.; Shaffrey, C.I.; et al. Less invasive surgery for treating adult spinal deformities: Ceiling effects for deformity correction with 3 different techniques. Neurosurg. Focus 2014, 36, E12. [Google Scholar] [CrossRef]
- Moller, D.J.; Slimack, N.P.; Acosta, F.L., Jr.; Koski, T.R.; Fessler, R.G.; Liu, J.C. Minimally invasive lateral lumbar interbody fusion and transpsoas approach-related morbidity. Neurosurg. Focus 2011, 31, E4. [Google Scholar] [CrossRef] [Green Version]
- Tempel, Z.J.; Gandhoke, G.S.; Bonfield, C.M.; Okonkwo, D.O.; Kanter, A.S. Radiographic and clinical outcomes following combined lateral lumbar interbody fusion and posterior segmental stabilization in patients with adult degenerative scoliosis. Neurosurg. Focus 2014, 36, E11. [Google Scholar] [CrossRef]
- Rodgers, W.B.; Gerber, E.J.; Patterson, J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: An analysis of 600 cases. Spine 2011, 36, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Lenke, L.G.; Shaffrey, C.I.; Van Alstyne, E.M.; Skelly, A.C. Proximal junctional kyphosis as a distinct form of adjacent segment pathology after spinal deformity surgery: A systematic review. Spine 2012, 37, S144–S164. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Akilah, K.B.; Boachie-Adjei, O. Incidence, risk factors and classification of proximal junctional kyphosis: Surgical outcomes review of adult idiopathic scoliosis. Spine 2011, 36, E60–E68. [Google Scholar] [CrossRef] [PubMed]
- Bridwell, K.H.; Lenke, L.G.; Cho, S.K.; Pahys, J.M.; Zebala, L.P.; Dorward, I.G.; Cho, W.; Baldus, C.; Hill, B.W.; Kang, M.M. Proximal junctional kyphosis in primary adult deformity surgery: Evaluation of 20 degrees as a critical angle. Neurosurgery 2013, 72, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Cahill, P.J.; Wang, W.; Asghar, J.; Booker, R.; Betz, R.R.; Ramsey, C.; Baran, G. The use of a transition rod may prevent proximal junctional kyphosis in the thoracic spine after scoliosis surgery: A finite element analysis. Spine 2012, 37, E687–E695. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Taniguchi, S.; Adachi, T.; Kushida, T.; Paku, M.; Ando, M.; Saito, T.; Kotani, Y.; Tani, Y. Rod contour and overcorrection are risk factors of proximal junctional kyphosis after adult spinal deformity correction surgery. Eur. Spine J. 2021, 30, 1208–1214. [Google Scholar] [CrossRef]
- Rhee, J.M.; Bridwell, K.H.; Won, D.S.; Lenke, L.G.; Chotigavanichaya, C.; Hanson, D.S. Sagittal plane analysis of adolescent idiopathic scoliosis: The effect of anterior versus posterior instrumentation. Spine 2002, 27, 2350–2356. [Google Scholar] [CrossRef]
- Korkmaz, M.; Akgul, T.; Sariyilmaz, K.; Ozkunt, O.; Dikici, F.; Yazicioglu, O. Effectiveness of posterior structures in the development of proximal junctional kyphosis following posterior instrumentation: A biomechanical study in a sheep spine model. Acta Orthop. Traumatol. Turc. 2019, 53, 385–389. [Google Scholar] [CrossRef]
- Lange, T.; Schmoelz, W.; Gosheger, G.; Eichinger, M.; Heinrichs, C.H.; Boevingloh, A.S.; Schulte, T.L. Is a gradual reduction of stiffness on top of posterior instrumentation possible with a suitable proximal implant? A biomechanical study. Spine J. 2017, 17, 1148–1155. [Google Scholar] [CrossRef]
- Barton, C.; Noshchenko, A.; Patel, V.; Cain, C.; Kleck, C.; Burger, E. Risk factors for rod fracture after posterior correction of adult spinal deformity with osteotomy: A retrospective case-series. Scoliosis 2015, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.S.; Shaffrey, E.; Klineberg, E.; Shaffrey, C.I.; Lafage, V.; Schwab, F.J.; Protopsaltis, T.; Scheer, J.K.; Mundis, G.M.; Fu, K.-M.G.; et al. Prospective multicenter assessment of risk factors for rod fracture following surgery for adult spinal deformity. J. Neurosurg. Spine 2014, 21, 994–1003. [Google Scholar] [CrossRef] [Green Version]
- Merrill, R.K.; Kim, J.S.; Leven, D.M.; Kim, J.H.; Cho, S.K. Multi-rod constructs can prevent rod breakage and pseudarthrosis at the lumbosacral junction in adult spinal deformity. Glob. Spine J. 2017, 7, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Izeki, M.; Fujio, K.; Ota, S.; Soga, S.; Matsuda, S.J. Radiological follow-up of the degenerated facet joints after lateral lumbar interbody fusion with percutaneous pedicle screw fixation: Focus on spontaneous facet joint fusion. J. Orthop. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.; Núñez-Pereira, S.; Pigrau, C.; Rodríguez-Pardo, D.; Vila-Casademunt, A.; Alanay, A.; Acaroglu, E.R.; Kleinstueck, F.S.; Obeid, I.; Perez-Grueso, F.J.S.; et al. The impact of deep surgical site infection on surgical outcomes after posterior adult spinal deformity surgery: A matched control study. Eur. Spine J. 2018, 27, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Uribe, J.S.; Deukmedjian, A.R.; Mummaneni, P.V.; Fu, K.M.; Mundis, G.M.; Okonkwo, D.O.; Kanter, A.S.; Eastlack, R.; Wang, M.Y.; Anand, N.; et al. Complications in adult spinal deformity surgery: An analysis of minimally invasive, hybrid, and open surgical techniques. Neurosurg. Focus 2014, 36, E15. [Google Scholar] [CrossRef]
- Hamilton, D.K.; Kanter, A.S.; Bolinger, B.D.; Mundis, G.M.; Nguyen, S.; Mummaneni, P.V.; Anand, N.; Fessler, R.G.; Passias, P.G.; Park, P.; et al. Reoperation rates in minimally invasive, hybrid and open surgical treatment for adult spinal deformity with minimum 2-year follow-up. Eur. Spine J. 2016, 25, 2605–2611. [Google Scholar] [CrossRef]




| Spinal Disease | Indication | |
|---|---|---|
| De novo kyphoscoliosis | GI | |
| Spinal deformity with OVF | GI (with corpectomy) | |
| Degenerative kyphosis | UI | |
| Adult scoliosis | without bone union | UI |
| anterior bone union | UI | |
| anterior and posterior bone union | UNI (with mini-open Ponte) | |
| Iatrogenic kyphosis | one level | UI |
| Multi-level | NI | |
| Parameter | Whole Group (n = 145) | Group G (n = 110) | Group P (n = 35) | |
|---|---|---|---|---|
| Age (years) | 73.3 ± 6.5 (48–83) | 73.3 ± 6.9 | 73.0 ± 7.7 | |
| Rate of women (%) | 88 | 80.8 | 78.1 | |
| Period of follow-up (months) | 40.7 ± 6.2 (30–54) | 40.7 ± 6.3 | 40.6 ± 6.2 | |
| Rod diameter/number in construct | 5.5 mm/2 rods | 54 (61%) | 40 | 14 |
| 6 mm/2 rods | 48 (30%) | 33 | 15 | |
| 5.5 mm/3 rods | 43 (1%) | 33 | 10 | |
| Number of levels fused | 10.3 ± 0.5 (10–13) | 10.3 ± 0.5 | 10.4 ± 05 | |
| Number of LLIF | 4.0 ± 0.5 (3–6) | 4.0 ± 03 | 4.1 ± 05 | |
| Number of patients with corpectomy(case) | 8 | 7 | 1 | |
| UIV (case) | T7 | 3 (1%) | 3 | 0 |
| T9 | 48 (29%) | 34 | 14 | |
| T10 | 94 (70%) | 72 | 22 | |
| Operative time (min) | Anterior (first surgery) | 109.6 ± 37.5 (59–273) | 112.4 ± 40.2 | 100.8 ± 26.5 |
| Posterior (second surgery) | 233.3 ± 50.9 ** (171–290) | 233.2 ± 52.1 ** | 233.0 ± 47.7 ** | |
| Blood loss (mL) | Anterior (first surgery) | 104.3 ± 139.3 (0–970) | 117.7 ± 152.4 | 63.0 ± 75.1 |
| Posterior (second surgery) | 498.2 ± 305.7 ** (79–1530) | 488.6 ± 290.9 ** | 528.1 ± 350.9 ** | |
| VAS back | Before surgery | 6.6 ± 1.4 | 6.4 ± 1.3 | 7.2 ± 1.2 |
| Final | 2.8 ± 0.8 * | 2.6 ± 0.7 *# | 3.3 ± 0.9 * | |
| VAS leg | Before surgery | 5.4 ± 2.2 | 5.5 ± 2.1 | 5.0 ± 2.6 |
| Final | 1.7 ± 1.0 * | 1.7 ± 1.1 * | 1.7 ± 1.1 * | |
| ODI | Before surgery | 37.9 ± 6.2 | 37.3 ± 6.2 | 40.1 ± 5.1 |
| Final | 22.1 ± 5.6 * | 20.8 ± 5.8 *# | 25.9 ± 2.6 * | |
| Parameter | Pre-Op | Post-Op | Final | p Value (Pre-Op vs. Final) |
|---|---|---|---|---|
| PI | 47.3 ± 10.5 | 48.0 ± 11.1 | 47.9 ± 10.8 | 0.542 |
| PI-LL | 37.3 ± 17.9 | 1.2 ± 12.2 | 2.7 ± 12.1 | <0.001 * |
| LL | 11.3 ± 15.9 | 48.3 ± 10.3 | 46.5 ± 10.8 | <0.001 * |
| PT | 31.8 ± 11.2 | 17.5 ± 9.8 | 18.5 ± 9.2 | <0.001 * |
| TK | 19.4 ± 16.3 | 39.2 ± 10.8 | 42.3 ± 11.6 | <0.001 * |
| SVA | 83.3 ± 50.1 | 15.7 ± 35.0 | 38.0 ± 36.5 | <0.001 * |
| Variable | Group P (n = 35) | Group G (n = 110) | p Value | |
|---|---|---|---|---|
| PI | Pre-PI | 57.1 ± 10.6 | 44.8 ± 10.4 | <0.001 * |
| Post-PI | 58.2 ± 10.7 | 46.5 ± 10.7 | <0.001 * | |
| LL | Pre-LL | 10.4 ± 13.8 | 11.5 ± 16.7 | 0.590 |
| LL after LLIF | 28.5 ± 10.4 | 33.3 ± 11.1 | 0.065 | |
| Post-LL | 41.4 ± 10.0 | 50.4 ± 9.5 | <0.001 * | |
| ΔLL | ΔLL (with LLIF) | 21.1 ± 11.2 | 21.7 ± 12.9 | 0.776 |
| ΔLL (with PPS) | 10.0 ± 8.8 | 17.1 ± 10.0 | 0.109 | |
| Total ΔLL | 31.1 ± 15.1 | 38.8 ± 16.7 | 0.210 | |
| PI-LL | Pre-PI-LL | 44.6 ± 17.1 | 35.0 ± 17.7 | <0.001 * |
| PI-LL after LLIF | 26.7 ± 7.7 | 13.1 ± 12.0 | <0.001 * | |
| Post-PI-LL | 16.7 ± 5.2 | −3.8 ± 8.8 | <0.001 * | |
| PT | Pre-PT | 37.6 ± 11.5 | 29.9 ± 10.6 | <0.001 * |
| Post-PT | 26.3 ± 7.6 | 14.8 ± 8.5 | <0.001 * | |
| TK | Pre-TK | 18.9 ± 15.2 | 19.6 ± 16.9 | 0.305 |
| Post-TK | 36.4 ± 8.8 | 37.5 ± 11.4 | 0.625 | |
| Odd Ratio | 95% CI | p Value | |
|---|---|---|---|
| Pre-op PI | 0.93 | 0.87–0.99 | 0.032 * |
| PI-LL after LLIF | 0.87 | 0.79–0.95 | 0.001 * |
| Pre-op PT | 0.98 | 0.90–1.06 | 0.618 |
| Pre-op PI-LL | 1.04 | 0.99–1.10 | 0.068 |
| Cut-Off Value | Sensitivity | Specificity | AUC | |
|---|---|---|---|---|
| PI-LL after LLIF | 20° | 0.95 | 0.65 | 0.846 |
| PI | 56° | 0.75 | 0.83 | 0.781 |
| n = 145 | ≤30 Days | 30 Days 3 Years | After 3 Years | Total | p Value | ||
|---|---|---|---|---|---|---|---|
| PJK | 5.5 mm, 2 rods (n = 54) (case) | 3 | 4 | 0 | 7 (13%) | n.s. | |
| 6 mm, 2 rods (n = 48) (case) | 2 | 6 | 0 | 8 (17%) | |||
| 5.5 mm, multi-rods (n = 43) (case) | 1 | 3 | 0 | 4 (9%) | |||
| Total (case) | 6 | 13 | 0 | 19 (13%) | |||
| Revision (case) | 3 | 9 | 0 | 12 (8%) | |||
| Rod fracture | Rod diameter, number | 5.5 mm, 2 rods (n = 54) (case) | 0 | 18 | 2 | 20 (37%) | 5.5 mm vs. 6 mm: 0.008 * 5.5 mm vs. multi: 0.001 * |
| 6 mm, 2 rods (n = 48) (case) | 0 | 7 | 0 | 7 (15%) | |||
| 5.5 mm, multi-rods (n = 43) (case) | 0 | 4 | 0 | 4 (9%) | |||
| Total (case) | 0 | 29 | 2 | 31 (21%) | |||
| Reasons | Nonunion (case) | 0 | 19 | 0 | 19 (13%) | ||
| ALL rupture (case) | 0 | 9 | 0 | 9 (6%) | |||
| After union (case) | 0 | 1 | 2 | 3 (2%) | |||
| Revision (case) | 0 | 28 | 0 | 28 (19%) | |||
| Neurological deficit (case) | 5 (transient 3, permanent 2) | 0 | 0 | 5 (3%) | |||
| Thigh symptom (case) | 56 (transient) | 0 | 0 | 56 (39%) | |||
| Infection (case) | 1 | 1 | 0 | 2 (1%) | |||
| Breakage of SAI (case) | 0 | 6 (revision 3) | 0 | 6 (4%) | |||
| Coronal imbalance (case) | 16 | 2 | 1 | 19 (13%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, M.; Taniguchi, S.; Adachi, T.; Tani, Y.; Paku, M.; Ando, M.; Saito, T. Conditions for Achieving Postoperative Pelvic Incidence-Lumbar Lordosis < 10° in Circumferential Minimally Invasive Surgery for Adult Spinal Deformity. J. Clin. Med. 2022, 11, 1586. https://doi.org/10.3390/jcm11061586
Ishihara M, Taniguchi S, Adachi T, Tani Y, Paku M, Ando M, Saito T. Conditions for Achieving Postoperative Pelvic Incidence-Lumbar Lordosis < 10° in Circumferential Minimally Invasive Surgery for Adult Spinal Deformity. Journal of Clinical Medicine. 2022; 11(6):1586. https://doi.org/10.3390/jcm11061586
Chicago/Turabian StyleIshihara, Masayuki, Shinichirou Taniguchi, Takashi Adachi, Yoichi Tani, Masaaki Paku, Muneharu Ando, and Takanori Saito. 2022. "Conditions for Achieving Postoperative Pelvic Incidence-Lumbar Lordosis < 10° in Circumferential Minimally Invasive Surgery for Adult Spinal Deformity" Journal of Clinical Medicine 11, no. 6: 1586. https://doi.org/10.3390/jcm11061586