Antimetastatic Effects of Sesamin on Human Head and Neck Squamous Cell Carcinoma through Regulation of Matrix Metalloproteinase-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Sesamin Treatment
2.3. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide) Assay
2.4. Culture Inserts for the Wound Healing Assay
2.5. Cell Migration and Invasion Assays
2.6. Cell Protein Preparation
2.7. Western Blot Assay
2.8. Statistical Analysis
3. Results
3.1. Cell Viability of Human Oral Cancer Cells after Sesamin Treatment
3.2. Motility of Sesamin-Treated Human Oral Cancer Cells According to Results of a Wound-Healing Assay
3.3. Invasion and Migration of Human Oral Cancer Cells after Sesamin Treatment
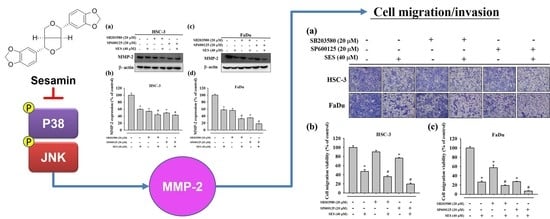
3.4. Effect of Sesamin on MMP-2 Expression in Human Oral Cancer Cells
3.5. Effect of Sesamin on the Association between MMP-2 and MAPK in Human Oral Cancer Cells
3.6. Effect of Combinatorial Treatment of MAPK Inhibitors and Sesamin on the Motility of Human Oral Cancer Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Hisamatsu, K.; Suzui, N.; Hara, A.; Tomita, H.; Miyazaki, T. A Review of HPV-Related Head and Neck Cancer. J. Clin. Med. 2018, 7, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer 2001, 94, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefebvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.W.; Chuang, C.Y.; Hsieh, Y.S.; Chen, P.N.; Yang, S.F.; Shih Hsuan, L.; Chen, Y.Y.; Lin, C.W.; Chang, Y.C. Rubus idaeus extract suppresses migration and invasion of human oral cancer by inhibiting MMP-2 through modulation of the Erk1/2 signaling pathway. Environ. Toxicol. 2017, 32, 1037–1046. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Su, C.W.; Chen, M.K.; Hung, W.C.; Yang, S.F.; Chuang, C.Y.; Lin, C.W. Functional variant of CHI3L1 gene is associated with neck metastasis in oral cancer. Clin. Oral. Investig. 2019, 23, 2685–2694. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Chen, J.C.; Yang, W.E.; Chien, S.Y.; Chen, M.K.; Lo, Y.S.; Hsi, Y.T.; Chuang, Y.C.; Lin, C.C.; Yang, S.F. Dehydroandrographolide inhibits oral cancer cell migration and invasion through NF-kappaB-, AP-1-, and SP-1-modulated matrix metalloproteinase-2 inhibition. Biochem. Pharmacol. 2017, 130, 10–20. [Google Scholar] [CrossRef]
- Hardy, E.; Hardy-Sosa, A.; Fernandez-Patron, C. MMP-2: Is too low as bad as too high in the cardiovascular system? Am. J. Physiol-Heart Circ. Physiol. 2018, 315, H1332–H1340. [Google Scholar] [CrossRef]
- Chen, M.; Gilbert, N.; Liu, H. Reduced expression of PD-L1 in autoimmune thyroiditis attenuate trophoblast invasion through ERK/MMP pathway. Reprod. Biol. Endocrinol. 2019, 17, 86. [Google Scholar] [CrossRef]
- Yeh, C.M.; Lin, C.W.; Yang, J.S.; Yang, W.E.; Su, S.C.; Yang, S.F. Melatonin inhibits TPA-induced oral cancer cell migration by suppressing matrix metalloproteinase-9 activation through the histone acetylation. Oncotarget 2016, 7, 21952–21967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.W.; Hsieh, Y.H.; Yang, W.E.; Yang, S.F.; Chen, Y.; Hu, D.N. Epigallocatechingallate inhibits migration of human uveal melanoma cells via downregulation of matrix metalloproteinase-2 activity and ERK1/2 pathway. Biomed. Res. Int. 2014, 2014, 141582. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Hsieh, M.J.; Hsieh, Y.S.; Chen, P.N.; Yang, J.S.; Lo, F.C.; Yang, S.F.; Lu, K.H. Tricetin inhibits human osteosarcoma cells metastasis by transcriptionally repressing MMP-9 via p38 and Akt pathways. Environ. Toxicol. 2017, 32, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Chin, M.C.; Lin, C.C.; His, Y.T.; Lo, Y.S.; Chuang, Y.C.; Chen, M.K. Pinostilbene Hydrate Suppresses Human Oral Cancer Cell Metastasis by Downregulation of Matrix Metalloproteinase-2 Through the Mitogen-Activated Protein Kinase Signaling Pathway. Cell Physiol. Biochem. 2018, 50, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Talvensaari-Mattila, A.; Paakko, P.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br. J. Cancer 2003, 89, 1270–1275. [Google Scholar] [CrossRef]
- Lin, T.Y.; Wu, P.Y.; Hou, C.W.; Chien, T.Y.; Chang, Q.X.; Wen, K.C.; Lin, C.Y.; Chiang, H.M. Protective Effects of Sesamin against UVB-Induced Skin Inflammation and Photodamage In Vitro and In Vivo. Biomolecules 2019, 9, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Gou, X.; Cai, P.; Xu, C.; Cao, L.; Zhao, Z.; Huang, M.; Jin, J. Sesamin Enhances Nrf2-Mediated Protective Defense against Oxidative Stress and Inflammation in Colitis via AKT and ERK Activation. Oxid. Med. Cell Longev. 2019, 2019, 2432416. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Massri, M.; Nasrallah, G.K. A comprehensive review on the anti-cancer properties and mechanisms of action of sesamin, a lignan in sesame seeds (Sesamum indicum). Eur. J. Pharmacol. 2017, 815, 512–521. [Google Scholar] [CrossRef]
- Siao, A.C.; Hou, C.W.; Kao, Y.H.; Jeng, K.C. Effect of sesamin on apoptosis and cell cycle arrest in human breast cancer mcf-7 cells. Asian Pac. J. Cancer Prev. 2015, 16, 3779–3783. [Google Scholar] [CrossRef] [Green Version]
- Deng, P.; Wang, C.; Chen, L.; Wang, C.; Du, Y.; Yan, X.; Chen, M.; Yang, G.; He, G. Sesamin induces cell cycle arrest and apoptosis through the inhibition of signal transducer and activator of transcription 3 signalling in human hepatocellular carcinoma cell line HepG2. Biol. Pharm. Bull. 2013, 36, 1540–1548. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, H.; Kuribayashi, K.; Tsuji, N.; Tanaka, M.; Kobayashi, D.; Watanabe, N. Sesamin induces autophagy in colon cancer cells by reducing tyrosine phosphorylation of EphA1 and EphB2. Int. J. Oncol. 2011, 39, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Pandey, M.K.; Joy, B.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Sesamin manifests chemopreventive effects through the suppression of NF-kappa B-regulated cell survival, proliferation, invasion, and angiogenic gene products. Mol. Cancer Res. 2010, 8, 751–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.L.; Kuo, F.H.; Chen, P.N.; Hsieh, Y.H.; Yu, N.Y.; Yang, W.E.; Hsieh, M.J.; Yang, S.F. Andrographolide suppresses the migratory ability of human glioblastoma multiforme cells by targeting ERK1/2-mediated matrix metalloproteinase-2 expression. Oncotarget 2017, 8, 105860–105872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beggs, J.E.; Tian, S.; Jones, G.G.; Xie, J.; Iadevaia, V.; Jenei, V.; Thomas, G.; Proud, C.G. The MAP kinase-interacting kinases regulate cell migration, vimentin expression and eIF4E/CYFIP1 binding. Biochem. J. 2015, 467, 63–76. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, Y.H.; Hsieh, M.J.; Yang, S.F.; Chen, S.P.; Tsai, W.C.; Chen, P.N. Phloretin suppresses metastasis by targeting protease and inhibits cancer stemness and angiogenesis in human cervical cancer cells. Phytomedicine 2019, 62, 152964. [Google Scholar] [CrossRef]
- Chen, M.K.; Liu, Y.T.; Lin, J.T.; Lin, C.C.; Chuang, Y.C.; Lo, Y.S.; Hsi, Y.T.; Hsieh, M.J. Pinosylvin reduced migration and invasion of oral cancer carcinoma by regulating matrix metalloproteinase-2 expression and extracellular signal-regulated kinase pathway. Biomedi. Pharmacother. 2019, 117, 109160. [Google Scholar] [CrossRef]
- Wang, K.; Yang, S.F.; Hsieh, Y.H.; Chang, Y.Y.; Yu, N.Y.; Lin, H.W.; Lin, H.Y. Effects of dihydromyricetin on ARPE-19 cell migration through regulating matrix metalloproteinase-2 expression. Environ. Toxicol. 2018, 33, 1298–1303. [Google Scholar] [CrossRef]
- Chien, S.Y.; Hsieh, M.J.; Chen, C.J.; Yang, S.F.; Chen, M.K. Nobiletin inhibits invasion and migration of human nasopharyngeal carcinoma cell lines by involving ERK1/2 and transcriptional inhibition of MMP-2. Expert Opin. Ther. Targets 2015, 19, 307–320. [Google Scholar] [CrossRef]
- Cooney, R.V.; Custer, L.J.; Okinaka, L.; Franke, A.A. Effects of dietary sesame seeds on plasma tocopherol levels. Nutr. Cancer 2001, 39, 66–71. [Google Scholar] [CrossRef]
- Schutten, J.C.; Joosten, M.M.; de Borst, M.H.; Bakker, S.J.L. Magnesium and Blood Pressure: A Physiology-Based Approach. Adv. Chronic. Kidney Dis. 2018, 25, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of Specific Dietary Fats with Total and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Mengxi, W.; Mo, L.; Renjie, Z.; Jing, W.; Dongya, C.; Hong, L. Ameliorating effect of sesamin on insulin resistance of hepatic L02 cells induced by high glucose/high insulin. Pak. J. Pharm. Sci. 2019, 32, 2733–2739. [Google Scholar]
- Pascual, G.; Dominguez, D.; Benitah, S.A. The contributions of cancer cell metabolism to metastasis. Dis. Model. Mech. 2018, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokota, T.; Matsuzaki, Y.; Koyama, M.; Hitomi, T.; Kawanaka, M.; Enoki-Konishi, M.; Okuyama, Y.; Takayasu, J.; Nishino, H.; Nishikawa, A.; et al. Sesamin, a lignan of sesame, down-regulates cyclin D1 protein expression in human tumor cells. Cancer Sci. 2007, 98, 1447–1453. [Google Scholar] [CrossRef]
- Dou, H.; Yang, S.; Hu, Y.; Xu, D.; Liu, L.; Li, X. Sesamin induces ER stress-mediated apoptosis and activates autophagy in cervical cancer cells. Life Sci. 2018, 200, 87–93. [Google Scholar] [CrossRef]
- Tseng, P.Y.; Liu, Y.T.; Lin, C.C.; Chuang, Y.C.; Lo, Y.S.; Hsi, Y.T.; Hsieh, M.J.; Chen, M.K. Pinostilbene Hydrate Inhibits the Migration and Invasion of Human Nasopharyngeal Carcinoma Cells by Downregulating MMP-2 Expression and Suppressing Epithelial-Mesenchymal Transition Through the Mitogen-Activated Protein Kinase Signaling Pathways. Front. Oncol. 2019, 9, 1364. [Google Scholar] [CrossRef] [Green Version]
- Hsin, C.H.; Huang, C.C.; Chen, P.N.; Hsieh, Y.S.; Yang, S.F.; Ho, Y.T.; Lin, C.W. Rubus idaeus Inhibits Migration and Invasion of Human Nasopharyngeal Carcinoma Cells by Suppression of MMP-2 through Modulation of the ERK1/2 Pathway. Am. J. Chin. Med. 2017, 45, 1557–1572. [Google Scholar] [CrossRef]
- Chung, H.H.; Chen, M.K.; Chang, Y.C.; Yang, S.F.; Lin, C.C.; Lin, C.W. Inhibitory effects of Leucaena leucocephala on the metastasis and invasion of human oral cancer cells. Environ. Toxicol. 2017, 32, 1765–1774. [Google Scholar] [CrossRef]
- Chao, R.; Chow, J.M.; Hsieh, Y.H.; Chen, C.K.; Lee, W.J.; Hsieh, F.K.; Yu, N.Y.; Chou, M.C.; Cheng, C.W.; Yang, S.F.; et al. Tricetin suppresses the migration/invasion of human glioblastoma multiforme cells by inhibiting matrix metalloproteinase-2 through modulation of the expression and transcriptional activity of specificity protein 1. Expert Opin. Ther. Targets 2015, 19, 1293–1306. [Google Scholar] [CrossRef]
- Cho, S.Y.; Klemke, R.L. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J. Cell Biol. 2000, 149, 223–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.T.; Hsieh, M.J.; Hsieh, Y.H.; Hsin, M.C.; Chuang, Y.T.; Yang, S.F.; Yang, J.S.; Lin, C.W. Sulforaphane suppresses oral cancer cell migration by regulating cathepsin S expression. Oncotarget 2018, 9, 17564–17575. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: No samples of the compounds are available from the authors. |








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-M.; Chen, P.-Y.; Lin, C.-C.; Hsieh, M.-C.; Lin, J.-T. Antimetastatic Effects of Sesamin on Human Head and Neck Squamous Cell Carcinoma through Regulation of Matrix Metalloproteinase-2. Molecules 2020, 25, 2248. https://doi.org/10.3390/molecules25092248
Chen J-M, Chen P-Y, Lin C-C, Hsieh M-C, Lin J-T. Antimetastatic Effects of Sesamin on Human Head and Neck Squamous Cell Carcinoma through Regulation of Matrix Metalloproteinase-2. Molecules. 2020; 25(9):2248. https://doi.org/10.3390/molecules25092248
Chicago/Turabian StyleChen, Jian-Ming, Pei-Yin Chen, Chia-Chieh Lin, Ming-Chang Hsieh, and Jen-Tsun Lin. 2020. "Antimetastatic Effects of Sesamin on Human Head and Neck Squamous Cell Carcinoma through Regulation of Matrix Metalloproteinase-2" Molecules 25, no. 9: 2248. https://doi.org/10.3390/molecules25092248