Focus on Formononetin: Anticancer Potential and Molecular Targets
Abstract
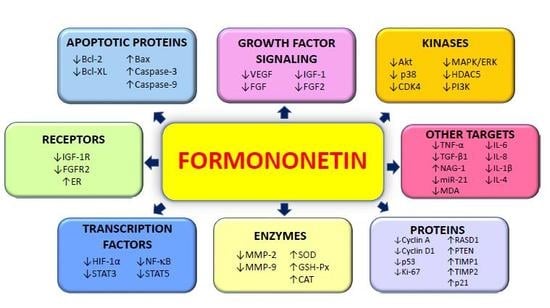
:1. Introduction
2. Toxicity and Pharmacokinetics of Formononetin
3. In Vitro Anticancer Pharmacological Properties of Formononetin
3.1. Antiproliferative Effects
3.2. Proapoptotic Effects
3.3. Induction of Cell Cycle Arrest
3.4. Antioxidant Effects
3.5. Angiogenesis-Modulating Effects
3.6. Metastasis-Regulatory Effects
3.7. Anti-Inflammatory Effects
3.8. Combinatorial Studies with Selected Chemotherapeutics
3.9. Novel Semi-Synthetic Hybrids of Formononetin
4. In Vivo Anticancer Pharmacological Activities of Formononetin
5. Formononetin in Clinical Studies
6. Conclusion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| AP-1 | activator protein 1 |
| Bax | Bcl-2-associated protein |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-xL | B-cell lymphoma-extra-large |
| b-FGF | basic fibroblast growth factor |
| CAT | catalase |
| CDK | cyclin-dependent kinase |
| CD4 | cluster of differentiation 4 |
| CREB | cAMP response element-binding protein |
| DMSO | dimethyl sulfoxide |
| EGR3 | early growth response protein 3 |
| EMT | epithelial-mesenchymal transition |
| ERK | extracellular signal regulated kinase |
| FGF | fibroblast growth factor |
| FGF2 | fibroblast growth factor 2 receptor |
| GSH-Px | glutathione peroxidase |
| GSK-3β | glycogen synthase kinase 3β |
| HDAC5 | histone deacetylase 5 |
| HIF-1α | hypoxia-inducible factor 1α |
| IGF-1 | insulin-like growth factor 1 |
| IGF-1R | insulin-like growth factor 1 receptor |
| IL-1β | interleukin 1β |
| IL-4 | interleukin-4 |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| MAPK | mitogen-activated protein kinase |
| MDA | malondialdehyde |
| miR-21 | microRNA 21 |
| MMP | matrix metalloproteinase |
| NAG-1 | NSAID-activated gene |
| NF-κB | nuclear factor-κB |
| PI3K | phosphatidylinositol 3-kinase |
| PTEN | phosphatase and tensin homolog |
| ROCK | Rho-associated protein kinase |
| siRNA | small interfering ribonucleic acid |
| SOD | superoxide dismutase |
| STAT3 | signal transducer and activator of transcription 3 |
| Sul-F | formononetin-3′-sulphonate |
| TMZ | temozolomide |
| TNF-α | tumor necrosis factor-α |
| VCAM-1 | vascular cell adhesion protein 1 |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
| Wnt | Wingless |
References
- Martin, T.A.; Ye, L.; Sanders, A.J.; Lane, J.; WG, J. Cancer invasion and metastasis: Molecular and cellular perspective. In Metastatic Cancer Clinical Biological Perspectives; Landes Bioscience 2000–2013: Austin, TX, USA, 2013. [Google Scholar] [CrossRef]
- Cooper, G.M. The development and causes of cancer. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: Globocan sources and methods. Int. J. Cancer 2018. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Kannaiyan, R.; Sethi, G. Targeting cell signaling and apoptotic pathways by dietary agents: Role in the prevention and treatment of cancer. Nutr. Cancer 2011, 63, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Johnston, S.; Corrie, P.; Kuhn, I.; Barclay, S. Withdrawal of anticancer therapy in advanced disease: A systematic literature review. BMC Cancer 2015, 15, 892. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: A systematic review of the world literature. BMC Med. 2016, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef]
- Neill, U.S. From branch to bedside: Youyou tu is awarded the 2011 lasker~debakey clinical medical research award for discovering artemisinin as a treatment for malaria. J. Clin. Investig. 2011, 121, 3768–3773. [Google Scholar] [CrossRef]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Deorukhkar, A.; Krishnan, S.; Sethi, G.; Aggarwal, B.B. Back to basics: How natural products can provide the basis for new therapeutics. Exp. Opin. Invest. Drugs 2007, 16, 1753–1773. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Weng, C.J.; Sethi, G.; Hu, D.N. Natural bioactives and phytochemicals serve in cancer treatment and prevention. Evid. Based Complement. Altern. Med. 2013, 2013, 698190. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Sethi, G.; Kuo, P.L. Novel medicines and strategies in cancer treatment and prevention. BioMed. Res. Int. 2014, 2014, 474078. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Yang, S.F.; Sethi, G.; Hu, D.N. Natural bioactives in cancer treatment and prevention. BioMed. Res. Int. 2015, 2015, 182835. [Google Scholar] [CrossRef]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 2016, 40–41, 48–81. [Google Scholar] [CrossRef]
- Hasanpourghadi, M.; Looi, C.Y.; Pandurangan, A.K.; Sethi, G.; Wong, W.F.; Mustafa, M.R. Phytometabolites targeting the warburg effect in cancer cells: A mechanistic review. Curr. Drug Targets 2017, 18, 1086–1094. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential role of natural compounds as anti-angiogenic agents in cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sethi, G.; Baladandayuthapani, V.; Krishnan, S.; Shishodia, S. Targeting cell signaling pathways for drug discovery: An old lock needs a new key. J. Cell Biochem. 2007, 102, 580–592. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Hwang, S.T.; Sethi, G.; Fan, L.; Arfuso, F.; Ahn, K.S. Potential anti-inflammatory and anti-cancer properties of farnesol. Molecules 2018, 23, 2827. [Google Scholar] [CrossRef]
- Merarchi, M.; Sethi, G.; Fan, L.; Mishra, S.; Arfuso, F.; Ahn, K.S. Molecular targets modulated by fangchinoline in tumor cells and preclinical models. Molecules 2018, 23, 2538. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Shanmugam, M.K.; Warrier, S.; Merarchi, M.; Arfuso, F.; Kumar, A.P.; Bishayee, A. Pro-apoptotic and anti-cancer properties of diosgenin: A comprehensive and critical review. Nutrients 2018, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Nabavi, S.F.; Nabavi, S.M.; Sureda, A.; Farooqi, A.A.; Atanasov, A.G.; Vacca, R.A.; Sethi, G.; Bishayee, A. Targeting activator protein 1 signaling pathway by bioactive natural agents: Possible therapeutic strategy for cancer prevention and intervention. Pharmacol. Res. 2018, 128, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016, 40–41, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.; Sethi, G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Shrimali, D.; Shanmugam, M.K.; Kumar, A.P.; Zhang, J.; Tan, B.K.; Ahn, K.S.; Sethi, G. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013, 341, 139–149. [Google Scholar] [CrossRef]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A neolignan from the magnolia family for the prevention and treatment of cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef]
- Ko, J.H.; Arfuso, F.; Sethi, G.; Ahn, K.S. Pharmacological utilization of bergamottin, derived from grapefruits, in cancer prevention and therapy. Int. J. Mol. Sci. 2018, 19, 4048. [Google Scholar] [CrossRef]
- Parikh, N.R.; Mandal, A.; Bhatia, D.; Siveen, K.S.; Sethi, G.; Bishayee, A. Oleanane triterpenoids in the prevention and therapy of breast cancer: Current evidence and future perspectives. Phytochem. Rev. 2014, 13, 793–810. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: Preclinical and clinical evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Li, F.; Shanmugam, M.K.; Kannaiyan, R.; Goh, J.N.; Wong, K.F.; Wang, W.; Khin, E.; Tergaonkar, V.; Kumar, A.P.; et al. Celastrol suppresses growth and induces apoptosis of human hepatocellular carcinoma through the modulation of stat3/jak2 signaling cascade in vitro and in vivo. Cancer Prev. Res. (Phila.) 2012, 5, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, R.; Hay, H.S.; Rajendran, P.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; et al. Celastrol inhibits proliferation and induces chemosensitization through down-regulation of nf-kappab and stat3 regulated gene products in multiple myeloma cells. Br. J. Pharmacol. 2011, 164, 1506–1521. [Google Scholar] [CrossRef] [PubMed]
- Akincilar, S.C.; Low, K.C.; Liu, C.Y.; Yan, T.D.; Oji, A.; Ikawa, M.; Li, S.; Tergaonkar, V. Quantitative assessment of telomerase components in cancer cell lines. FEBS Lett. 2015, 589, 974–984. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Ko, J.K. Novel herbal flavonoids promote apoptosis but differentially induce cell cycle arrest in human colon cancer cell. Invest. New Drugs 2010, 28, 1–13. [Google Scholar] [CrossRef]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Miao, H.; Zhao, Y.; Kang, X.; Shang, S.; Xiang, W.; Shi, R.; Hou, A.; Wang, R.; Zhao, K.; et al. NF-κB potentiates tumor growth by suppressing a novel target lpts. Cell Commun. Signal. 2017, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Arora, L.; Kumar, A.P.; Arfuso, F.; Chng, W.J.; Sethi, G. The role of signal transducer and activator of transcription 3 (STAT3) and its targeted inhibition in hematological malignancies. Cancers 2018, 10. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Lee, S.G.; Sethi, G.; Ahn, K.S. Ophiopogonin d, a steroidal glycoside abrogates STAT3 signaling cascade and exhibits anti-cancer activity by causing GSH/GSSG imbalance in lung carcinoma. Cancers 2018, 10. [Google Scholar] [CrossRef]
- Wong, A.L.A.; Hirpara, J.L.; Pervaiz, S.; Eu, J.Q.; Sethi, G.; Goh, B.C. Do stat3 inhibitors have potential in the future for cancer therapy? Expert Opin. Investig. Drugs 2017, 26, 883–887. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Baek, S.H.; Ko, J.H.; Lee, S.G.; Yang, W.M.; Um, J.Y.; Sethi, G.; Ahn, K.S. Capsazepine inhibits jak/stat3 signaling, tumor growth, and cell survival in prostate cancer. Oncotarget 2017, 8, 17700–17711. [Google Scholar] [CrossRef]
- Zhang, J.; Ahn, K.S.; Kim, C.; Shanmugam, M.K.; Siveen, K.S.; Arfuso, F.; Samym, R.P.; Deivasigamanim, A.; Lim, L.H.; Wang, L.; et al. Nimbolide-induced oxidative stress abrogates stat3 signaling cascade and inhibits tumor growth in transgenic adenocarcinoma of mouse prostate model. Antioxid. Redox Signal. 2016, 24, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Chai, E.Z.; Shanmugam, M.K.; Arfuso, F.; Dharmarajan, A.; Wang, C.; Kumar, A.P.; Samy, R.P.; Lim, L.H.; Wang, L.; Goh, B.C.; et al. Targeting transcription factor stat3 for cancer prevention and therapy. Pharmacol. Ther. 2016, 162, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.; Shanmugam, M.K.; Ong, T.H.; Li, F.; Perumal, E.; Chen, L.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; et al. Emodin inhibits growth and induces apoptosis in an orthotopic hepatocellular carcinoma model by blocking activation of stat3. Br. J. Pharmacol. 2013, 170, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.; Shanmugam, M.K.; Perumal, E.; Li, F.; Nachiyappan, A.; Dai, X.; Swamy, S.N.; Ahn, K.S.; Kumar, A.P.; Tan, B.K.; et al. Potential role of signal transducer and activator of transcription (stat)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim. Biophys. Acta 2013, 1835, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Lee, J.H.; Nam, D.; Narula, A.S.; Namjoshi, O.A.; Blough, B.E.; Um, J.Y.; Sethi, G.; Ahn, K.S. Anti-myeloma effects of icariin are mediated through the attenuation of jak/stat3-dependent signaling cascade. Front. Pharmacol. 2018, 9, 531. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.G.; Yang, W.M.; Arfuso, F.; Um, J.Y.; Kumar, A.P.; Bian, J.; Sethi, G.; Ahn, K.S. Formononetin-induced oxidative stress abrogates the activation of stat3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018, 431, 123–141. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Nf-kappab in cancer therapy. Arch. Toxicol. 2015, 89, 711–731. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Fong, C.W.; Kumar, A.P.; Tan, P.; Sethi, G. First evidence that gamma-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of nf-kappab pathway. Clin. Cancer Res. 2012, 18, 2220–2229. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Lee, J.H.; Kannaiyan, R.; Mustafa, N.; Manu, K.A.; Siveen, K.S.; Sethi, G.; Chng, W.J.; Kumar, A.P. Celastrol attenuates the invasion and migration and augments the anticancer effects of bortezomib in a xenograft mouse model of multiple myeloma. Front. Pharmacol. 2018, 9, 365. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Siveen, K.S.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Arfuso, F.; Kumar, A.P.; et al. Isorhamnetin augments the anti-tumor effect of capeciatbine through the negative regulation of nf-kappab signaling cascade in gastric cancer. Cancer Lett. 2015, 363, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shanmugam, M.K.; Siveen, K.S.; Wang, F.; Ong, T.H.; Loo, S.Y.; Swamy, M.M.; Mandal, S.; Kumar, A.P.; Goh, B.C.; et al. Garcinol sensitizes human head and neck carcinoma to cisplatin in a xenograft mouse model despite downregulation of proliferative biomarkers. Oncotarget 2015, 6, 5147–5163. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Bharathkumar, H.; Dukanya; Rangappa, S.; Shanmugam, M.K.; Chinnathambi, A.; Alharbi, S.A.; Alahmadi, T.A.; Bhattacharjee, A.; Lobie, P.E.; et al. N-substituted pyrido-1,4-oxazin-3-ones induce apoptosis of hepatocellular carcinoma cells by targeting nf-kappab signaling pathway. Front. Pharmacol. 2018, 9, 1125. [Google Scholar] [PubMed]
- Raghunath, A.; Sundarraj, K.; Arfuso, F.; Sethi, G.; Perumal, E. Dysregulation of NRF2 in hepatocellular carcinoma: Role in cancer progression and chemoresistance. Cancers 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Ko, J.H.; Jung, Y.Y.; Jung, S.H.; Kim, E.; Kong, M.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; et al. Casticin inhibits growth and enhances ionizing radiation-induced apoptosis through the suppression of stat3 signaling cascade. J. Cell Biochem. 2019, 120, 9787–9798. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone inhibits bone metastasis of breast cancer cells through abrogation of the cxcr4 signaling axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Alharbi, S.A.; Chang, Y.; Sethi, G.; et al. Oleuropein induces apoptosis via abrogating nf-kappab activation cascade in estrogen receptor-negative breast cancer cells. J. Cell Biochem. 2019, 120, 4504–4513. [Google Scholar] [CrossRef]
- Li, F.; Sethi, G. Targeting transcription factor nf-kappab to overcome chemoresistance and radioresistance in cancer therapy. Biochim. Biophys. Acta 2010, 1805, 167–180. [Google Scholar]
- Sethi, G.; Tergaonkar, V. Potential pharmacological control of the nf-kappab pathway. Trends. Pharmacol. Sci. 2009, 30, 313–321. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Aggarwal, B.B. Nuclear factor-kappa b: From clone to clinic. Curr. Mol. Med. 2007, 7, 619–637. [Google Scholar] [CrossRef]
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the involvement of the master transcription factor nf-kappab in cancer initiation and progression. Biomedicines 2018, 6. [Google Scholar] [CrossRef]
- Chai, E.Z.; Siveen, K.S.; Shanmugam, M.K.; Arfuso, F.; Sethi, G. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem. J. 2015, 468, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.Y.; Arya, A.; Naema, A.F.; Wong, W.F.; Sethi, G.; Looi, C.Y. Signal transducer and activator of transcription (stats) proteins in cancer and inflammation: Functions and therapeutic implication. Front. Oncol. 2019, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Hirpara, J.L.; Eu, J.Q.; Sethi, G.; Wang, L.; Goh, B.C.; Wong, A.L. Targeting stat3 and oxidative phosphorylation in oncogene-addicted tumors. Redox Biol. 2018, 101073. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tergaonkar, V.; Krishna, S.; Androphy, E.J. Human papillomavirus type 16 e6-enhanced susceptibility of l929 cells to tumor necrosis factor alpha correlates with increased accumulation of reactive oxygen species. J. Biol. Chem. 1999, 274, 24819–24827. [Google Scholar] [CrossRef]
- Akincilar, S.C.; Khattar, E.; Boon, P.L.; Unal, B.; Fullwood, M.J.; Tergaonkar, V. Long-range chromatin interactions drive mutant tert promoter activation. Cancer Discov. 2016, 6, 1276–1291. [Google Scholar] [CrossRef]
- Khattar, E.; Kumar, P.; Liu, C.Y.; Akincilar, S.C.; Raju, A.; Lakshmanan, M.; Maury, J.J.; Qiang, Y.; Li, S.; Tan, E.Y.; et al. Telomerase reverse transcriptase promotes cancer cell proliferation by augmenting trna expression. J. Clin. Investig. 2016, 126, 4045–4060. [Google Scholar] [CrossRef]
- Mishra, S.; Verma, S.S.; Rai, V.; Awasthee, N.; Chava, S.; Hui, K.M.; Kumar, A.P.; Challagundla, K.B.; Sethi, G.; Gupta, S.C. Long non-coding rnas are emerging targets of phytochemicals for cancer and other chronic diseases. Cell Mol. Life Sci. 2019, 76, 1947–1966. [Google Scholar] [CrossRef]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef]
- Siveen, K.S.; Sikka, S.; Surana, R.; Dai, X.; Zhang, J.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Targeting the stat3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim. Biophys. Acta 2014, 1845, 136–154. [Google Scholar] [CrossRef]
- Yang, M.H.; Jung, S.H.; Sethi, G.; Ahn, K.S. Pleiotropic pharmacological actions of capsazepine, a synthetic analogue of capsaicin, against various cancers and inflammatory diseases. Molecules 2019, 24, 995. [Google Scholar] [CrossRef] [PubMed]
- Girisa, S.; Shabnam, B.; Monisha, J.; Fan, L.; Halim, C.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Kunnumakkara, A.B. Potential of zerumbone as an anti-cancer agent. Molecules 2019, 24, 734. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Um, J.Y.; Sethi, G.; Ahn, K.S. Casticin-induced inhibition of cell growth and survival are mediated through the dual modulation of akt/mtor signaling cascade. Cancers 2019, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Kashyap, D.; Sak, K.; Tuli, H.S.; Jain, A.; Chaudhary, A.; Garg, V.K.; Sethi, G.; Yerer, M.B. Molecular mechanisms of action of tocotrienols in cancer: Recent trends and advancements. Int. J. Mol. Sci. 2019, 20, 656. [Google Scholar] [CrossRef] [PubMed]
- Polak, R.; Phillips, E.M.; Campbell, A. Legumes: Health benefits and culinary approaches to increase intake. Clin. Diabetes 2015, 33, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Cassileth, B. Complementary therapies, herbs, and other otc agents: Red clover (trifolium pratense). Oncology (Williston Park) 2010, 24, 960. [Google Scholar] [PubMed]
- Kaczmarczyk-Sedlak, I.; Wojnar, W.; Zych, M.; Ozimina-Kaminska, E.; Taranowicz, J.; Siwek, A. Effect of formononetin on mechanical properties and chemical composition of bones in rats with ovariectomy-induced osteoporosis. Evid. Based Complement. Altern. Med. 2013, 2013, 457052. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef] [PubMed]
- Lukaczer, D.; Darland, G.; Tripp, M.; Liska, D.; Lerman, R.H.; Schiltz, B.; Bland, J.S. Clinical effects of a proprietary combination isoflavone nutritional supplement in menopausal women: A pilot trial. Altern. Ther. Health Med. 2005, 11, 60–65. [Google Scholar]
- Hidalgo, L.A.; Chedraui, P.A.; Morocho, N.; Ross, S.; San Miguel, G. The effect of red clover isoflavones on menopausal symptoms, lipids and vaginal cytology in menopausal women: A randomized, double-blind, placebo-controlled study. Gynecol. Endocrinol. 2005, 21, 257–264. [Google Scholar] [CrossRef]
- Atkinson, C.; Compston, J.E.; Day, N.E.; Dowsett, M.; Bingham, S.A. The effects of phytoestrogen isoflavones on bone density in women: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2004, 79, 326–333. [Google Scholar] [CrossRef]
- Nestel, P.J.; Pomeroy, S.; Kay, S.; Komesaroff, P.; Behrsing, J.; Cameron, J.D.; West, L. Isoflavones from red clover improve systemic arterial compliance but not plasma lipids in menopausal women. J. Clin. Endocrinol. Metab. 1999, 84, 895–898. [Google Scholar] [CrossRef]
- Wang, A.L.; Li, Y.; Zhao, Q.; Fan, L.Q. Formononetin inhibits colon carcinoma cell growth and invasion by microrna149mediated ephb3 downregulation and inhibition of pi3k/akt and stat3 signaling pathways. Mol. Med. Rep. 2018, 17, 7721–7729. [Google Scholar] [PubMed]
- Zhang, S.; Tang, X.; Tian, J.; Li, C.; Zhang, G.; Jiang, W.; Zhang, Z. Cardioprotective effect of sulphonated formononetin on acute myocardial infarction in rats. Basic Clin. Pharmacol. Toxicol. 2011, 108, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zou, L.; Tian, J.; Lin, F.; He, J.; Hou, J. Protective effects of sulphonated formononetin in a rat model of cerebral ischemia and reperfusion injury. Planta Med. 2014, 80, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Tolleson, W.H.; Doerge, D.R.; Churchwell, M.I.; Marques, M.M.; Roberts, D.W. Metabolism of biochanin a and formononetin by human liver microsomes in vitro. J. Agric. Food Chem. 2002, 50, 4783–4790. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.H.; Cho, D.; Kim, T.S. Formononetin, a phyto-oestrogen, and its metabolites up-regulate interleukin-4 production in activated t cells via increased ap-1 DNA binding activity. Immunology 2005, 116, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, J.; Chen, X.; Li, J.; Shen, M.; Yu, W.; Yang, Y.; Xiao, Z. The proapoptotic effect of formononetin in human osteosarcoma cells: Involvement of inactivation of erk and akt pathways. Cell Physiol. Biochem. 2014, 34, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zeng, J.; Xin, M.; Huang, W.; Chen, X. Formononetin induces cell cycle arrest of human breast cancer cells via igf1/pi3k/akt pathways in vitro and in vivo. Horm. Metab. Res. 2011, 43, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, L. Formononetin-induced apoptosis by activation of ras/p38 mitogen-activated protein kinase in estrogen receptor-positive human breast cancer cells. Horm. Metab. Res. 2012, 44, 943–948. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, X.; Ye, Y.; Wang, Y.; Tian, J. Estrogen receptor beta-mediated proliferative inhibition and apoptosis in human breast cancer by calycosin and formononetin. Cell Physiol. Biochem. 2013, 32, 1790–1797. [Google Scholar] [CrossRef]
- Ye, Y.; Hou, R.; Chen, J.; Mo, L.; Zhang, J.; Huang, Y.; Mo, Z. Formononetin-induced apoptosis of human prostate cancer cells through erk1/2 mitogen-activated protein kinase inactivation. Horm. Metab. Res. 2012, 44, 263–267. [Google Scholar] [CrossRef]
- Li, T.; Zhao, X.; Mo, Z.; Huang, W.; Yan, H.; Ling, Z.; Ye, Y. Formononetin promotes cell cycle arrest via downregulation of akt/cyclin d1/cdk4 in human prostate cancer cells. Cell Physiol. Biochem. 2014, 34, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, Y.; Ai, X.; Cheng, B.; Lu, S. Formononetin suppresses the proliferation of human non-small cell lung cancer through induction of cell cycle arrest and apoptosis. Int. J. Clin. Exp. Pathol. 2014, 7, 8453–8461. [Google Scholar]
- Jin, Y.M.; Xu, T.M.; Zhao, Y.H.; Wang, Y.C.; Cui, M.H. In vitro and in vivo anti-cancer activity of formononetin on human cervical cancer cell line HELA. Tumour Biol. 2014, 35, 2279–2284. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Li, Z.; Yan, H.; Qin, J.; Li, T. Formononetin inhibits human bladder cancer cell proliferation and invasiveness via regulation of mir-21 and pten. Food Funct. 2017, 8, 1061–1066. [Google Scholar] [CrossRef]
- Park, S.; Bazer, F.W.; Lim, W.; Song, G. The o-methylated isoflavone, formononetin, inhibits human ovarian cancer cell proliferation by sub g0/g1 cell phase arrest through PI3K/AKT and ERK1/2 inactivation. J. Cell Biochem. 2018, 119, 7377–7387. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Li, H.Y.; Wang, R.H.; Ma, X.X.; Yue, B.; Yan, J.; Yang, N.L.; Xing, X.H.; Sheng, Z.F.; Wang, G.H.; et al. Formononetin suppresses hypoxia inducible factor-1α/inflammatory cytokines expression via inhibiting akt signal pathway in multiple myeloma cells. Int. J. Clin. Exp. Med. 2016, 9, 1117–1127. [Google Scholar]
- Huang, J.; Xie, M.; Gao, P.; Ye, Y.; Liu, Y.; Zhao, Y.; Luo, W.; Ling, Z.; Cao, Y.; Zhang, S.; et al. Antiproliferative effects of formononetin on human colorectal cancer via suppressing cell growth in vitro and in vivo. Proc. Biochem. 2015, 50, 912–917. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Aapro, M.S.; Plezia, P.M.; Alberts, D.S.; Graham, V.; Jones, S.E.; Surwit, E.A.; Moon, T.E. Double-blind crossover study of the antiemetic efficacy of high-dose dexamethasone versus high-dose metoclopramide. J. Clin. Oncol. 1984, 2, 466–471. [Google Scholar] [CrossRef]
- Huang, W.J.; Bi, L.Y.; Li, Z.Z.; Zhang, X.; Ye, Y. Formononetin induces the mitochondrial apoptosis pathway in prostate cancer cells via downregulation of the igf-1/igf-1r signaling pathway. Pharm. Biol. 2013, 52, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Xiao, Z. Formononetin induces apoptosis of human osteosarcoma cell line u2os by regulating the expression of bcl-2, bax and mir-375 in vitro and in vivo. Cell Physiol. Biochem. 2015, 37, 933–939. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, Y.Q.; Chen, Q.Y.; Xiao, S.J.; Zeng, S.E. Up-regulating of rasd1 and apoptosis of du-145 human prostate cancer cells induced by formononetin in vitro. Asian Pac. J. Cancer Prev. 2014, 15, 2835–2839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bi, L.; Ye, Y.; Chen, J. Formononetin induces apoptosis in pc-3 prostate cancer cells through enhancing the bax/bcl-2 ratios and regulating the p38/akt pathway. Nutr. Cancer 2014, 66, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Xu, L.; Ye, M.; Liao, M.; Du, H.; Chen, H. Formononetin inhibits migration and invasion of mda-mb-231 and 4t1 breast cancer cells by suppressing mmp-2 and mmp-9 through pi3k/akt signaling pathways. Horm. Metab. Res. 2014, 46, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, K.K.; Law, P.C.; Ko, J.K. Novel anti-angiogenic effects of formononetin in human colon cancer cells and tumor xenograft. Oncol. Rep. 2012, 28, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ni, Q.; Wang, Y.; Fan, H.; Li, Y. Synergistic anticancer effects of formononetin and temozolomide on glioma c6 cells. Biol. Pharm. Bull 2018, 41, 1194–1202. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Zheng, J.M.; Yan, X.L.; Chen, H.M.; Chen, J.K.; Huang, H.Q. Formononetin sensitizes glioma cells to doxorubicin through preventing EMT via inhibition of histone deacetylase 5. Int. J. Clin. Exp. Pathol. 2015, 8, 6434–6441. [Google Scholar]
- Qi, C.; Xie, M.; Liang, J.; Li, H.; Li, Z.; Shi, S.; Yang, X.; Wang, Z.; Tang, J.; Tang, A. Formononetin targets the mapk and pi3k/akt pathways to induce apoptosis in human nasopharyngeal carcinoma cells in vitro and in vivo. Int. J. Clin. Exp. Med. 2016, 9, 1180–1189. [Google Scholar]
- Rosse, T.; Olivier, R.; Monney, L.; Rager, M.; Conus, S.; Fellay, I.; Jansen, B.; Borner, C. Bcl-2 prolongs cell survival after bax-induced release of cytochrome c. Nature 1998, 391, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wu, X.; Tang, J.; Xiao, N.; Zhao, G.; Zhang, L.; Ou, L. In vitro and in vivo studies of antiosteosarcoma activities of formononetin. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Montagut, C.; Settleman, J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009, 283, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Manning, B.D.; Cantley, L.C. Targeting the pi3k-akt pathway in human cancer: Rationale and promise. Cancer Cell 2003, 4, 257–262. [Google Scholar] [CrossRef]
- Gelain, A.; Mori, M.; Meneghetti, F.; Villa, S. Signal transducer and activator of transcription protein 3 (stat3): An update on its direct inhibitors as promising anticancer agents. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Avalle, L.; Camporeale, A.; Camperi, A.; Poli, V. Stat3 in cancer: A double edged sword. Cytokine 2017, 98, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Lee, J.; Um, J.Y.; Sethi, G.; Ahn, K.S. Arctiin is a pharmacological inhibitor of stat3 phosphorylation at tyrosine 705 residue and potentiates bortezomib-induced apoptotic and anti-angiogenic effects in human multiple myeloma cells. Phytomedicine 2018, 55, 282–292. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Shanmugam, M.K.; Narula, A.S.; Kim, C.; Lee, J.H.; Namjoshi, O.A.; Blough, B.E.; Sethi, G.; Ahn, K.S. Oxymatrine attenuates tumor growth and deactivates stat5 signaling in a lung cancer xenograft model. Cancers 2019, 11. [Google Scholar] [CrossRef]
- Wu, X.Y.; Xu, H.; Wu, Z.F.; Chen, C.; Liu, J.Y.; Wu, G.N.; Yao, X.Q.; Liu, F.K.; Li, G.; Shen, L. Formononetin, a novel fgfr2 inhibitor, potently inhibits angiogenesis and tumor growth in preclinical models. Oncotarget 2015, 6, 44563–44578. [Google Scholar] [CrossRef]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y.; et al. Ginkgolic acid inhibits invasion and migration and tgf-beta-induced emt of lung cancer cells through pi3k/akt/mtor inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef]
- Mohan, C.D.; Srinivasa, V.; Rangappa, S.; Mervin, L.; Mohan, S.; Paricharak, S.; Baday, S.; Li, F.; Shanmugam, M.K.; Chinnathambi, A.; et al. Trisubstituted-imidazoles induce apoptosis in human breast cancer cells by targeting the oncogenic pi3k/akt/mtor signaling pathway. PLoS ONE 2016, 11, e0153155. [Google Scholar] [CrossRef]
- Singh, S.S.; Yap, W.N.; Arfuso, F.; Kar, S.; Wang, C.; Cai, W.; Dharmarajan, A.M.; Sethi, G.; Kumar, A.P. Targeting the pi3k/akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015, 21, 12261–12273. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of akt/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, S.M.; Bae, H.; Nam, D.; Lee, J.H.; Lee, S.G.; Shim, B.S.; Kim, S.H.; Ahn, K.S.; Choi, S.H.; et al. Embelin inhibits growth and induces apoptosis through the suppression of akt/mtor/s6k1 signaling cascades. Prostate 2013, 73, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Nam, D.; Yun, H.M.; Lee, S.G.; Jang, H.J.; Sethi, G.; Cho, S.K.; Ahn, K.S. Beta-caryophyllene oxide inhibits growth and induces apoptosis through the suppression of pi3k/akt/mtor/s6k1 pathways and ros-mediated mapks activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Lee, S.G.; Yang, W.M.; Um, J.Y.; Sethi, G.; Ahn, K.S. Ophiopogonin d modulates multiple oncogenic signaling pathways, leading to suppression of proliferation and chemosensitization of human lung cancer cells. Phytomedicine 2018, 40, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Nam, D.; Um, J.Y.; Jung, S.H.; Sethi, G.; Ahn, K.S. Bergamottin suppresses metastasis of lung cancer cells through abrogation of diverse oncogenic signaling cascades and epithelial-to-mesenchymal transition. Molecules 2018, 23, 1601. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Baek, S.H.; Lee, J.H.; Kim, C.; Ko, J.H.; Lee, S.G.; Chinnathambi, A.; Alharbi, S.A.; Yang, W.M.; Um, J.Y.; et al. Isorhynchophylline, a potent plant alkaloid, induces apoptotic and anti-metastatic effects in human hepatocellular carcinoma cells through the modulation of diverse cell signaling cascades. Int. J. Mol. Sci. 2017, 18, 1095. [Google Scholar] [CrossRef]
- Kannaiyan, R.; Manu, K.A.; Chen, L.; Li, F.; Rajendran, P.; Subramaniam, A.; Lam, P.; Kumar, A.P.; Sethi, G. Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-jun n-terminal kinase and suppression of pi3 k/akt signaling pathways. Apoptosis 2011, 16, 1028–1041. [Google Scholar] [CrossRef]
- Sethi, G.; Ahn, K.S.; Sung, B.; Kunnumakkara, A.B.; Chaturvedi, M.M.; Aggarwal, B.B. Sh-5, an akt inhibitor potentiates apoptosis and inhibits invasion through the suppression of anti-apoptotic, proliferative and metastatic gene products regulated by ikappabalpha kinase activation. Biochem. Pharmacol. 2008, 76, 1404–1416. [Google Scholar] [CrossRef]
- Ma, X.; Hu, Y. Targeting pi3k/akt/mtor cascade: The medicinal potential, updated research highlights and challenges ahead. Curr. Med. Chem. 2013, 20, 2991–3010. [Google Scholar] [CrossRef] [PubMed]
- Dreher, D.; Junod, A.F. Role of oxygen free radicals in cancer development. Eur. J. Cancer 1996, 32A, 30–38. [Google Scholar] [CrossRef]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch. Biochem. Biophys. 1998, 356, 133–141. [Google Scholar] [CrossRef]
- Kubota, Y. Tumor angiogenesis and anti-angiogenic therapy. Keio J. Med. 2012, 61, 47–56. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, S.; Hao, J.; Yuan, X.; Luo, W.; Jiang, L.; Hu, Y.; Fu, Z.; Zhang, Y.; Zou, C. Endostar attenuates melanoma tumor growth via its interruption of b-fgf mediated angiogenesis. Cancer Lett. 2015, 359, 148–154. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, H.S.; Chng, W.J.; Tergaonkar, V. Activation of mutant tert promoter by ras-erk signaling is a key step in malignant progression of braf-mutant human melanomas. Proc. Natl. Acad. Sci. USA 2016, 113, 14402–14407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, Y.; Gao, L.; Yin, H.; Xie, Z.; Wang, D.; Zhu, Z.; Han, X. Formononetin attenuates il-1beta-induced apoptosis and NF-κB activation in ins-1 cells. Molecules 2012, 17, 10052–10064. [Google Scholar] [CrossRef]
- Duan, P.; Wang, Z.M. Clinical study on effect of astragalus in efficacy enhancing and toxicity reducing of chemotherapy in patients of malignant tumor. Zhongguo Zhong Xi Yi Jie He Za Zhi 2002, 22, 515–517. [Google Scholar]
- Van den Bent, M.J.; Taphoorn, M.J.; Brandes, A.A.; Menten, J.; Stupp, R.; Frenay, M.; Chinot, O.; Kros, J.M.; van der Rijt, C.C.; Vecht Ch, J.; et al. Phase ii study of first-line chemotherapy with temozolomide in recurrent oligodendroglial tumors: The european organization for research and treatment of cancer brain tumor group study 26971. J. Clin. Oncol. 2003, 21, 2525–2528. [Google Scholar] [CrossRef]
- Liu, X.; Fan, D. The epithelial-mesenchymal transition and cancer stem cells: Functional and mechanistic links. Curr. Pharm. Des. 2015, 21, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, J.; Zhang, X.; Shen, J.; Deng, L.; Liu, Q.; Li, G. Targeting sim2-s decreases glioma cell invasion through mesenchymal--epithelial transition. J. Cell Biochem. 2014, 115, 1900–1907. [Google Scholar]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- watsuki, M.; Mimori, K.; Yokobori, T.; Ishi, H.; Beppu, T.; Nakamori, S.; Baba, H.; Mori, M. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010, 101, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xu, H.J.; Cheng, H.; Xin, W.Q.; Chen, X.; Hu, K. Synthesis and antitumor activity of formononetin nitrogen mustard derivatives. Eur. J. Med. Chem. 2012, 54, 175–187. [Google Scholar] [CrossRef]
- Fu, D.J.; Zhang, L.; Song, J.; Mao, R.W.; Zhao, R.H.; Liu, Y.C.; Hou, Y.H.; Li, J.H.; Yang, J.J.; Jin, C.Y.; et al. Design and synthesis of formononetin-dithiocarbamate hybrids that inhibit growth and migration of pc-3 cells via mapk/wnt signaling pathways. Eur. J. Med. Chem. 2017, 127, 87–99. [Google Scholar] [CrossRef]
- Lin, H.Y.; Sun, W.X.; Zheng, C.S.; Han, H.W.; Wang, X.; Zhang, Y.H.; Qiu, H.Y.; Tang, C.Y.; Qi, J.L.; Lu, G.H.; et al. Synthesis, characterization and biological evaluation of formononetin derivatives as novel egfr inhibitors via inhibiting growth, migration and inducing apoptosis in breast cancer cell line. RSC Adv. 2017, 7, 48404–48419. [Google Scholar] [CrossRef]
- Li, Y.Q.; Yang, F.; Wang, L.; Cao, Z.; Han, T.J.; Duan, Z.A.; Li, Z.; Zhao, W.J. Phosphoramidate protides of five flavones and their antiproliferative activity against hepg2 and l-o2 cell lines. Eur. J. Med. Chem. 2016, 112, 196–208. [Google Scholar] [CrossRef]
- Yang, C.; Xie, Q.; Zeng, X.; Tao, N.; Xu, Y.; Chen, Y.; Wang, J.; Zhang, L. Novel hybrids of podophyllotoxin and formononetin inhibit the growth, migration and invasion of lung cancer cells. Bioorg. Chem. 2019, 85, 445–454. [Google Scholar] [CrossRef]
- Guo, B.; Liao, C.; Liu, X.; Yi, J. Preliminary study on conjugation of formononetin with multiwalled carbon nanotubes for inducing apoptosis via ros production in hela cells. Drug Des. Dev. Ther. 2018, 12, 2815–2826. [Google Scholar] [CrossRef]
- Mu, H.; Bai, Y.H.; Wang, S.T.; Zhu, Z.M.; Zhang, Y.W. Research on antioxidant effects and estrogenic effect of formononetin from trifolium pratense (red clover). Phytomedicine 2009, 16, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.X.; Liu, X.L.; Xue, C.K.; Huang, Q.; Liu, Z.G.; Xiong, L. The estrogenic effect of formononetin and its effect on the expression of rats’ atrium estrogen receptors. Zhong Yao Cai 2010, 33, 1445–1449. [Google Scholar] [PubMed]
- Bertolini, F.; Marighetti, P.; Martin-Padura, I.; Mancuso, P.; Hu-Lowe, D.D.; Shaked, Y.; D’Onofrio, A. Anti-vegf and beyond: Shaping a new generation of anti-angiogenic therapies for cancer. Drug Discov. Today 2011, 16, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Sadek, I. Sunitinib: The antiangiogenic effects and beyond. Oncol. Targets Ther. 2016, 9, 5495–5505. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.X.; Zhang, Y.B.; Liu, T.; Zhang, X.J.; Wu, S.L. Neuroprotective effect of formononetin in ameliorating learning and memory impairment in mouse model of alzheimer’s disease. Biosci. Biotechnol. Biochem. 2018, 82, 57–64. [Google Scholar] [CrossRef]
- Jarred, R.A.; Keikha, M.; Dowling, C.; McPherson, S.J.; Clare, A.M.; Husband, A.J.; Pedersen, J.S.; Frydenberg, M.; Risbridger, G.P. Induction of apoptosis in low to moderate-grade human prostate carcinoma by red clover-derived dietary isoflavones. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1689–1696. [Google Scholar]



| Cancer Type/Cell Line Used | Concentration | Anticancer Effect | Mechanisms of Action | References |
|---|---|---|---|---|
| Bladder cancer | ||||
| T24 cell line | 50–200 μM | Antiproliferative Anti-invasion | ↑Apoptosis; ↑PTEN; ↓miR-21; ↓pAKT | [98] |
| MCF-7 cell line | 30–100 μM | Antiproliferative | ↑Apoptosis; ↑G0/G1 cell cycle arrest; ↓IGF-1/IGFR-PI3K/AKT pathway | [91] |
| Breast cancer | ||||
| ER-positive MCF-7 cells and T47D cell | 25–100 μM | Antiproliferative | ↑Apoptosis; ↓p38MAPK pathway | [92] |
| ER-positive MCF-7 cells and T47D cell | 25–100 μM | Antiproliferative | ↑Caspase-3; ↓IGF1R; ↓miR375 | [93] |
| MDA-MB-231 4TI | 2.5–40 μmol/L | Antiproliferative | ↓MMP-2; ↓MMP-9, ↓TIMP1; ↓TIMP2; ↓PI3K/AKT pathway | [108] |
| Cervical cancer | ||||
| HeLa cells | Not available | Antiproliferative | ↑Apoptosis; ↓PI3K/AKT pathway; ↓ERK pathway | [97] |
| Colon cancer | ||||
| LoVo | 50 μM | Anti-invasion | ↑Apoptosis; ↓VEGF; ↓MMP | [109] |
| Colorectal cancer | ||||
| HCT116 cell line | 6.25–200 μM | Antiproliferative | ↑Apoptosis; ↑Bax; ↑NAG-1; ↓Bcl-2; ↓Bcl-xL | [37] |
| SW1116 cell line HCT116 cell line | 20–200 μM | Antiproliferative | ↑miR-149; ↓EphB3; ↓PI3K/AKT pathway; ↓STAT3 pathway | [85] |
| RKO cell line | 20–80 μM | Antiproliferative | ↑Apoptosis; ↓ERK pathway | [101] |
| Glioma | ||||
| Glioma C6 cell line | 20–320 μM | Antiproliferative | ↑Apoptosis; ↑Bax; ↑cleaved caspase-3 & caspase-9; ↓Bcl-2; ↓MMP-2; ↓MMP-9 | [110] |
| Glioblastoma | ||||
| U87MG cell line U251MG cell line T98G cell line | 50–200 μM | Antiproliferative | ↓HDAC5; ↓doxorubicin-induced EMT | [111] |
| Multiple myeloma | ||||
| U266 cell line | 5–60 μM | Antiproliferative | ↓HIF-1α; ↓inflammatory cytokines; ↓AKT pathway | [100] |
| Nasopharyngeal carcinoma | ||||
| CNE1 cell line CNE2 cell line | 5–40 μM | ↓Cell viability | ↑Apoptosis; ↓PI3K/AKT pathway; ↓ERK pathway | [112] |
| Non-small cell lung cancer | ||||
| A549 cell line NCI-H23 cell line | 100–200 μM | Antiproliferative | ↑Apoptosis; ↑caspase-3; ↑Bax; ↓Bcl-2; ↑P21; ↓cyclin A; ↓cyclin D1; ↑G1 cell cycle arrest; ↑p53 at Ser15 and Ser20 | [96] |
| Osteosarcoma | ||||
| U2OS | 20–80 μM | Antiproliferative | ↑Apoptosis; ↑caspase-3; ↑Bax; ↓Bcl-2; ↓PI3K/AKT pathway; ↓ERK pathway | [90] |
| Ovarian cancer | ||||
| ES2 cell line OV90 cell line | 20–40 µM | Antiproliferative | ↑Apoptosis; ↑G0/G1 cell cycle arrest | [99] |
| Prostate cancer | ||||
| LNCaP cell line PC-3 cell line | 20–80 µM | Antiproliferative | ↑Apoptosis; ↑G1 cell cycle arrest; ↓AKT/cyclin D1/CDK4; ↓ERK1/2 pathway | [94] |
| PC-3 cell line DU-145 cell line | 10–100 µM | Antiproliferative | ↑Apoptosis; ↑G1/S cell cycle arrest; ↓IGF/IGFR1 pathway | [95] |
| PC-3 cell line | 25–100 µM | Antiproliferative | ↑Apoptosis; ↓IGF/IGFR1 pathway | [104] |
| DU-145 cell line | 6.25–200 μM | Antiproliferative | ↑Apoptosis; ↑Bax; ↑RASD1; ↑caspase-3; ↑PARP; ↓Bcl-2 | [106] |
| PC-3 cell line | 25–100 μM | Antiproliferative | ↑Bax/Bcl-2 ratio; ↓p38MAPK/AKT pathway | [107] |
| Cancer Model | Dose, Duration and Route of Administration | Observed Effects | Mechanisms | References |
|---|---|---|---|---|
| MCF-7 cells-induced xenograft in Balb/c nude mice | 60 mg/kg/day; 20 days; i.p. | ↓Tumor growth | ↓IGF-1/IGFR-PI3K/AKT pathway | [91] |
| MDA-MB-231 cells-induced xenografts in Balb/c nude mice | 100 mg/kg/day; 25 days; intra-gastric (i.g.) | ↓Tumor growth (synergistic effect with sunitinib) | ↓FGF2-induced angiogenesis; ↓PI3K/AKT pathway; ↓STAT3 pathway | [121] |
| MDA-MB-231-luc cells-induced xenografts in Balb/c nude mice | 10 or 20 mg/kg/day; once every 2 days for 35 days; i.p. | ↓Tumor growth; ↓lung metastasis; ↑overall survival | ↓PI3K/AKT pathway | [108] |
| HCT-116 cells-induced xenografts in Balb/c nu/nu mice | 20 mg/kg/day; 2 weeks; i.p. | ↓Tumor growth | ↓Tumor angiogenesis; ↓VEGF | [109] |
| Human multiple myeloma U266 xenograft in Balb/c nude mice | 20 and 50 mg/kg/day; 25 days; i.g. | ↓Tumor growth | ↓PI3K/AKT pathway | [92] |
| Human multiple myeloma tumor tissues implanted in athymic nu/nu mice | 40 mg/kg; thrice/week for 3 weeks; i.p. | ↓Tumor growth | ↓Tumor angiogenesis; ↓STAT3/5 pathway; ↓VEGF | [49] |
| PC-3 cells-induced prostate xenograft in nude mice | 60 mg/kg/day; 20 days; i.p. | ↓Tumor growth | ↑Apoptosis; ↓IGF/IGFR1 pathway | [95] |
| RKO tumor-bearing Balb/c nude mice | 5, 10 or 20 mg/kg/day; 14 days; i.g. | ↓Tumor growth | ↓IL6; ↓TNF-α; ↓NF-κB pathway | [101] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, S.K.L.; Shanmugam, M.K.; Fan, L.; Fraser, S.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Bishayee, A. Focus on Formononetin: Anticancer Potential and Molecular Targets. Cancers 2019, 11, 611. https://doi.org/10.3390/cancers11050611
Ong SKL, Shanmugam MK, Fan L, Fraser SE, Arfuso F, Ahn KS, Sethi G, Bishayee A. Focus on Formononetin: Anticancer Potential and Molecular Targets. Cancers. 2019; 11(5):611. https://doi.org/10.3390/cancers11050611
Chicago/Turabian StyleOng, Samantha Kah Ling, Muthu K. Shanmugam, Lu Fan, Sarah E. Fraser, Frank Arfuso, Kwang Seok Ahn, Gautam Sethi, and Anupam Bishayee. 2019. "Focus on Formononetin: Anticancer Potential and Molecular Targets" Cancers 11, no. 5: 611. https://doi.org/10.3390/cancers11050611