Oligometastatic Pancreatic Cancer to the Liver in the Era of Neoadjuvant Chemotherapy: Which Role for Conversion Surgery? A Systematic Review and Meta-Analysis
Abstract
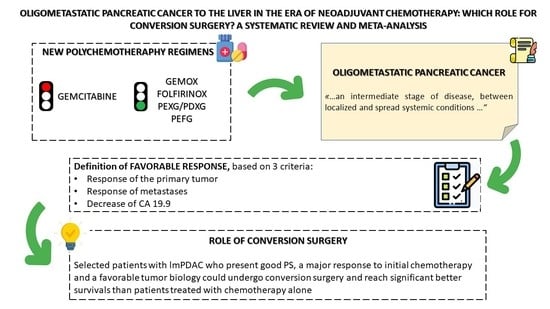
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Selection Criteria and Outcome Measures
2.4. Quality Assessment of Retrieved Articles
2.5. Data Extraction
2.6. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics and Patients Characteristics
3.3. Meta-Analysis: Survival Analysis
3.4. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclosure
References
- Howlader, N. SEER Cancer Statistics Review, 1975–2014. Available online: https://seer.cancer.gov/archive/csr/1975_2014/ (accessed on 22 January 2020).
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Facts & Figures. 2016. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html (accessed on 22 January 2020).
- Gillen, S.; Schuster, T.; Meyer zum Büschenfelde, C.; Friess, H.; Kleeff, J. Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages. PLoS Med. 2010, 7, e1000267. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Ahuja, N.; Makary, M.A.; Cameron, J.L.; Eckhauser, F.E.; Choti, M.A.; Hruban, R.H.; Pawlik, T.M.; Wolfgang, C.L. 2564 resected periampullary adenocarcinomas at a single institution: Trends over three decades. HPB 2014, 16, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louvet, C.; Philip, P.A. Accomplishments in 2007 in the treatment of metastatic pancreatic cancer. Gastrointest Cancer Res. 2008, 2, S37–S41. [Google Scholar] [PubMed]
- Disibio, G.; French, S.W. Metastatic patterns of cancers: Results from a large autopsy study. Arch. Pathol. Lab. Med. 2008, 132, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Isawa, T.; Koike, M.; Tsuruta, K.; Okamoto, A. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas 1995, 11, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Embuscado, E.E.; Laheru, D.; Ricci, F.; Yun, K.J.; de Boom Witzel, S.; Seigel, A.; Flickinger, K.; Hidalgo, M.; Bova, G.S.; Iacobuzio-Donahue, C.A. Immortalizing the complexity of cancer metastasis: Genetic features of lethal metastatic pancreatic cancer obtained from rapid autopsy. Cancer Biol. Ther. 2005, 4, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef]
- Burris, H.A.; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef] [Green Version]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Ghidini, M.; Petrillo, A.; Salati, M.; Khakoo, S.; Varricchio, A.; Tomasello, G.; Grossi, F.; Petrelli, F. Surgery or Locoregional Approaches for Hepatic Oligometastatic Pancreatic Cancer: Myth, Hope, or Reality? Cancers 2019, 11, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneda, H.; Saito, Y. Oligometastases: Defined by prognosis and evaluated by cure. Cancer Treat. Commun. 2015, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, S.W. Systemic Chemotherapy in Advanced Pancreatic Cancer. Gut Liver 2016, 10, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Rice, J.; Sharma, D.; Martin, R.C.G. The use of IRE in multi-modality treatment for oligometastatic pancreatic cancer. Am. J. Surg. 2018, 216, 106–110. [Google Scholar] [CrossRef]
- Schneitler, S.; Kröpil, P.; Riemer, J.; Antoch, G.; Knoefel, W.T.; Häussinger, D.; Graf, D. Metastasized pancreatic carcinoma with neoadjuvant FOLFIRINOX therapy and R0 resection. World J. Gastroenterol. 2015, 21, 6384–6390. [Google Scholar] [CrossRef]
- Hackert, T.; Sachsenmaier, M.; Hinz, U.; Schneider, L.; Michalski, C.W.; Springfeld, C.; Strobel, O.; Jäger, D.; Ulrich, A.; Büchler, M.W. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy with Folfirinox Results in Resectability in 60% of the Patients. Ann. Surg. 2016, 264, 457–463. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frigerio, I.; Regi, P.; Giardino, A.; Scopelliti, F.; Girelli, R.; Bassi, C.; Gobbo, S.; Martini, P.T.; Capelli, P.; D’Onofrio, M.; et al. Downstaging in Stage IV Pancreatic Cancer: A New Population Eligible for Surgery? Ann. Surg. Oncol. 2017, 24, 2397–2403. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Bittoni, A.; Sebastiani, E.; Partelli, S.; Zanon, S.; Lanese, A.; Andrikou, K.; Muffatti, F.; Balzano, G.; Reni, M.; et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur. J. Surg. Oncol. 2016, 42, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Heckler, M.; Mihaljevic, A.L.; Sun, H.; Klaiber, U.; Heger, U.; Büchler, M.W.; Hackert, T. CT response of primary tumor and CA19-9 predict resectability of metastasized pancreatic cancer after FOLFIRINOX. Eur. J. Surg. Oncol. 2019, 45, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Kandel, P.; Wallace, M.B.; Stauffer, J.; Bolan, C.; Raimondo, M.; Woodward, T.A.; Gomez, V.; Ritter, A.W.; Asbun, H.; Mody, K. Survival of Patients with Oligometastatic Pancreatic Ductal Adenocarcinoma Treated with Combined Modality Treatment Including Surgical Resection: A Pilot Study. J. Pancreat. Cancer 2018, 4, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, G.P.; Poruk, K.E.; Zenati, M.S.; Steve, J.; Bahary, N.; Hogg, M.E.; Zuriekat, A.H.; Wolfgang, C.L.; Zeh, H.J.; Weiss, M.J. Primary Tumor Resection Following Favorable Response to Systemic Chemotherapy in Stage IV Pancreatic Adenocarcinoma with Synchronous Metastases: A Bi-institutional Analysis. J. Gastrointest. Surg. 2016, 20, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Han, Y.; Kang, J.S.; Choi, Y.J.; Kim, H.; Kwon, W.; Kim, S.-W.; Oh, D.-Y.; Lee, S.H.; Ryu, J.K.; et al. Role of surgical resection in the era of FOLFIRINOX for advanced pancreatic cancer. J. Hepatobiliary Pancreat. Sci. 2019, 26, 416–425. [Google Scholar] [CrossRef]
- Moore, M.J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J.R.; Gallinger, S.; Au, H.J.; Murawa, P.; Walde, D.; Wolff, R.A.; et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 1960–1966. [Google Scholar] [CrossRef]
- Shrikhande, S.V.; Kleeff, J.; Reiser, C.; Weitz, J.; Hinz, U.; Esposito, I.; Schmidt, J.; Friess, H.; Büchler, M.W. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2007, 14, 118–127. [Google Scholar] [CrossRef]
- Tang, K.; Lu, W.; Qin, W.; Wu, Y. Neoadjuvant therapy for patients with borderline resectable pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. Pancreatology 2016, 16, 28–37. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Ohtsuka, T.; Kimura, R.; Matsuda, R.; Mori, Y.; Nakata, K.; Kakihara, D.; Fujimori, N.; Ohno, T.; Oda, Y.; et al. Neoadjuvant Chemotherapy with Gemcitabine Plus Nab-Paclitaxel for Borderline Resectable Pancreatic Cancer Potentially Improves Survival and Facilitates Surgery. Ann. Surg. Oncol. 2019, 26, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, C.R.; Marchegiani, G.; Hong, T.S.; Ryan, D.P.; Deshpande, V.; McDonnell, E.I.; Sabbatino, F.; Santos, D.D.; Allen, J.N.; Blaszkowsky, L.S.; et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann. Surg. 2015, 261, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilimoria, K.Y.; Bentrem, D.J.; Ko, C.Y.; Tomlinson, J.S.; Stewart, A.K.; Winchester, D.P.; Talamonti, M.S. Multimodality therapy for pancreatic cancer in the U.S.: Utilization, outcomes, and the effect of hospital volume. Cancer 2007, 110, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The “seed and soil” hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Shinozaki, K.; Yamada, T.; Nasu, J.; Matsumoto, T.; Yuasa, Y.; Shiraishi, T.; Nagano, H.; Moriyama, I.; Fujiwara, T.; Miguchi, M.; et al. A phase II study of FOLFOXIRI plus bevacizumab as initial chemotherapy for patients with untreated metastatic colorectal cancer: TRICC1414 (BeTRI). Int. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Gao, G.; Shen, L.; Xie, J.; Qian, X.; Wang, H. Should anti-EGFR mAbs be discontinued for conversion surgery in untreated right-sided metastatic colorectal cancer? A systematic review and meta-analysis. World J. Surg. Oncol. 2018, 16, 200. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, H.; Miyamoto, Y.; Higashi, T.; Hiyoshi, Y.; Yamao, T.; Uemura, N.; Matsumura, K.; Imai, K.; Yamashita, Y.-I.; Baba, H. CD44 expression enhances chemoresistance and implies occult micrometastases after conversion hepatectomy for initially unresectable colorectal liver metastases. Am. J. Transl. Res. 2020, 12, 5955–5966. [Google Scholar] [PubMed]
- Mielko, J.; Rawicz-Pruszyński, K.; Skórzewska, M.; Ciseł, B.; Pikuła, A.; Kwietniewska, M.; Gęca, K.; Sędłak, K.; Kurylcio, A.; Polkowski, W.P. Conversion Surgery with HIPEC for Peritoneal Oligometastatic Gastric Cancer. Cancers 2019, 11, 1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tachezy, M.; Gebauer, F.; Janot, M.; Uhl, W.; Zerbi, A.; Montorsi, M.; Perinel, J.; Adham, M.; Dervenis, C.; Agalianos, C.; et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery 2016, 160, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Reyes, D.K.; Pienta, K.J. The biology and treatment of oligometastatic cancer. Oncotarget 2015, 6, 8491–8524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagani, O.; Senkus, E.; Wood, W.; Colleoni, M.; Cufer, T.; Kyriakides, S.; Costa, A.; Winer, E.P.; Cardoso, F.; ESO-MBC Task Force. International guidelines for management of metastatic breast cancer: Can metastatic breast cancer be cured? J. Natl. Cancer Inst. 2010, 102, 456–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| References | Publication Year | Country | Inclusion Period | Type of Study | Study Design | No. of Patients Analyzed |
|---|---|---|---|---|---|---|
| Frigerio [24] | 2017 | Italy | 2007–2015 | Retrospective | Case series, two centers | 535 |
| Crippa [25] | 2016 | Italy | 2003–2013 | Retrospective | Case series, two centers | 127 |
| Tanaka [26] | 2019 | Germany | 2001–2017 | Retrospective | Case series, one center | 101 |
| Kandel [27] | 2018 | USA | 2005–2015 | Retrospective | Case series, one center | 42 |
| Wright [28] | 2016 | USA | 2008–2013 | Retrospective | Case series, two centers | 1147 |
| Byun [29] | 2019 | Japan | 2011–2017 | Retrospective | Case series, one center | 337 |
| Authors, Publication Year | Total Patients | Accurate Description of Surgical Procedure | Accurate Description of Chemotherapy Treatment | Consecutive | Newcastle−Ottawa Score | |||
|---|---|---|---|---|---|---|---|---|
| Selection Maximum **** | Comparability Maximum * | Outcome Maximum *** | Score (Out of 8) | |||||
| Frigerio [24], 2017 | 535 | Yes | Yes | Yes | *** | - | *** | 6 |
| Crippa [25], 2016 | 127 | Yes | Yes | Yes | *** | - | *** | 6 |
| Tanaka [26], 2019 | 101 | Yes | Yes | Yes | **** | * | *** | 8 |
| Kandel [27], 2018 | 42 | Yes | No | Yes | **** | * | *** | 8 |
| Wright [28], 2016 | 1147 | Yes | Yes | Yes | **** | * | *** | 8 |
| Byun [29], 2019 | 337 | No | Yes | Yes | **** | * | *** | 8 |
| References | Patients, N | Definition of Oligometastatic Disease or Inclusion Criteria for Surgery after IC | Patients Undergoing Surgery after IC, N (%) | Type of IC in Patients Undergoing Surgery, N (%) | Median Interval last IC-Surgery, Months (range) | Main Metastatic Localisation | Type of Surgery for Primary Lesion, N | Type of Surgery for Liver Metastases N | Adjuvant CT N (%) | 30 Day Mortality N (%) | OS Months (Range) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver, N | Lung, N | Lymphnodes, N | Peritoneum, N | |||||||||||
| Frigerio [24] | 535 | Disappearance of liver metastasis on radiological examination | 24 (4) | FOLFIRINOX N 16 (66), GEM N 5 (21), Gemcitabine + Nab-Paclitaxel N 3 (13) | 2 (2–16) *** | 24 | 0 | 0 | 0 | PD N 14 DP N 10 | None | N 15 (63) | Not specified | 56 (36–75) ** |
| Crippa [25] | 127 | Single metastasis remaining after initial chemotherapy | 11 (8) | FOLFIRINOX N3 (27), GEMOX N2 (2), PDXG N1 (9), PEFG N1 (9), PEXG N4 (36) | 12 (6–20) *** | 11 | 0 | 0 | 0 | PD N 6 DP N 5 | Atypical resection N 1, Segmentectomy N 2 | Not specified | Not specified | 39 * |
| Tanaka [26] | 101 | Maximum of six metastatic lesions. | 43 (42) | FOLFIRINOX N43 (100) | Not specified | 30 | 1 | 3 | 7 | PD N 16 DP N 19 TP N 8 | Not specified | N 4 (9), Unknown 9 (21) | 1 (2) | 21.9 (12.7–20.5) *** |
| Kandel [27] | 42 | ≤2 metastatic tumors total in liver or lung, each <4 cm | 6 (14) | Not specified | Not specified | 4 | 0 | 0 | 2 | PD N 4 DP N 2 | Hepatic resection N1, Hepatic resection and RFA n 2, Radioembolization N 1 | 6 (100) | 0 | 32/4 (1.4–44.28) ** |
| Wright [28] | 1147 | Not specified | 23 (2) | FOLFIRINOX N14 (60.9), Gemcitabine based regimens N 9 (39.1) | 9.7 (5.8–12.8) *** | 16 | 6 | 0 | 2 | PD 15 DP 8 | Metastasectomy N 9 | Not specified | 0 | 18.2 (11.8–35.5) *** |
| Byun [29] | 135 | Single metastatic lesion or resectable lesion | 8 (5) | FOLFIRINOX 8 (100) | Not specified | NS | NS | NS | NS | Not specified | Not specified | Not specified | Not specified | 32 * |
| Criteria of Response to IC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| References | Patients | Primary Tumor | Liver Metastases | Level of CA19.9 | Total Patients with lmPDAC, N | Patients Undergoing Surgery after IC, N (%) | Type of Initial Chemotherapy, N (%) | Complications after Surgery N (%) | Median Postoperative Stay Days (Range) | OS in Patients who Underwent IC+Surgery Months (Range) | OS in Patients who Underwent IC alone Months (Range) |
| Frigerio [24] (2017) | lmPDAC who responded to systemic chemotherapy and underwent successful surgery. | Not specified | Disappearance of liver metastasis on radiological examination | Normalization or significant reduction of CA19.9 | 535 | 24 (4.5) | FOLFIRINOX N 16 (66), GEM N 5 (21), Gemcitabine+Paclitaxel N 3 (13) | PF B N4 (16.5) PF C N1 (4) HEMORRHAGE N1 (4) SEPSIS N3 (12.5) | 13 (7–40) | 56 (36–75)°° | - |
| Crippa [25] (2016) | lmPDAC who responded to systemic chemotherapy and underwent successful surgery. | Resectable or borderline | Complete or a major radiological response of the liver metastases with a single metastasis remaining | Major biochemical response | 127 | 11 (8.5) | FOLFIRINOX N3 (27), PDXG N1 (9), PEFG N1 (9), PEXG N4 (36), GEMOX N2 (2) | PF A N2 | 8 | 39°° | 11°° |
| Kandel [27] (2018) | lmPDAC who underwent systemic chemotherapy, primary tumor resection, and metastasectomy and/or RFA | Not specified | Not specified | Not specified | Not specified | 4 (28) | Not specified | Not specified | Not specified | 23.25°° | 11.76°° |
| Tanaka [26] (2019) | lmPDAC undergoing pancreasectomy and metastasectomy after FOLFIRINOX | No tumor progression, technically resectable disease (resectable or borderline resectable, as defined by the NCCN guidelines) | A maximum of six metastatic lesions | Not specified | 57 | 30 (52.6) | All patients FOLFIRINOX | Not specified | 13 (5–56) | 25.39°° | 16.4°° |
| Byun [29] (2019) | lmPDAC who responded to systemic chemotherapy and underwent successful surgery. | Response to initial chemotherapy per RECIST criteria (Version 1.1), absence of local tumor extension to the major vessel | Single metastatic lesion or considered as resectable | Tumor markers normalized (or metabolic uptake decreased in PET) | 80 | 1 (1.25) | All patients FOLFIRINOX | None | Not specified | 23°° | 13°° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Simoni, O.; Scarpa, M.; Tonello, M.; Pilati, P.; Tolin, F.; Spolverato, Y.; Gruppo, M. Oligometastatic Pancreatic Cancer to the Liver in the Era of Neoadjuvant Chemotherapy: Which Role for Conversion Surgery? A Systematic Review and Meta-Analysis. Cancers 2020, 12, 3402. https://doi.org/10.3390/cancers12113402
De Simoni O, Scarpa M, Tonello M, Pilati P, Tolin F, Spolverato Y, Gruppo M. Oligometastatic Pancreatic Cancer to the Liver in the Era of Neoadjuvant Chemotherapy: Which Role for Conversion Surgery? A Systematic Review and Meta-Analysis. Cancers. 2020; 12(11):3402. https://doi.org/10.3390/cancers12113402
Chicago/Turabian StyleDe Simoni, Ottavia, Marco Scarpa, Marco Tonello, Pierluigi Pilati, Francesca Tolin, Ylenia Spolverato, and Mario Gruppo. 2020. "Oligometastatic Pancreatic Cancer to the Liver in the Era of Neoadjuvant Chemotherapy: Which Role for Conversion Surgery? A Systematic Review and Meta-Analysis" Cancers 12, no. 11: 3402. https://doi.org/10.3390/cancers12113402