The Role of Probiotics in Cancer Prevention
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Use of Probiotics in the Chemoprevention of Cancer
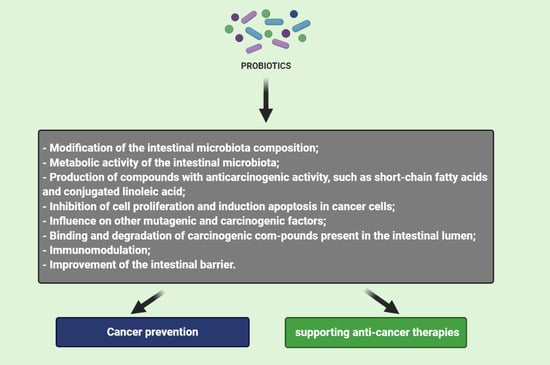
3. Mechanism of Probiotics Action in Cancer Prevention and Therapy
3.1. Modification of the Intestinal Microbiota Composition
3.2. Metabolic Activity of the Intestinal Microbiota
3.3. Production of Compounds with Anticarcinogenic Activity, Such as Short-Chain Fatty Acids and Conjugated Linoleic Acid
3.4. Inhibition of Cell Proliferation and Induction Apoptosis in Cancer Cells
3.5. Influence on Other Mutagenic and Carcinogenic Factors
3.6. Binding and Degradation of Carcinogenic Compounds Present in the Intestinal Lumen
3.7. Immunomodulation
3.8. Improvement of the Intestinal Barrier
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liśkiewicz, P.; Pełka-Wysiecka, J.; Wroński, M.; Bąba-Kubiś, A.; Samochowiec, J. Intestinal flora and the pathophysiology of depression and anxiety disorders—Current state of the art and future perspectives. Psychiatry 2018, 15, 70–76. [Google Scholar]
- Gonzalez, A.; Stombaugh, J.; Lozupone, C.; Turnbaugh, P.J.; Gordon, J.I.; Knight, R. The mind-body-microbial continuum. Dialogues. Clin. Neurosci. 2011, 13, 55–62. [Google Scholar]
- Snyder, L.; Peter, J.E.; Henkin, T.M.; Champress, W. Molecular Genetics of Bacteria, 4th ed.; ASM Press: Washington, DC, USA, 1997; pp. 53–66. [Google Scholar]
- Joseph, N.; Vasodavan, K.; Saipudin, N.A.; Yusof, B.N.M.; Kumar, S.; Nordin, S.A. Gut microbiota and short-chain fatty acids (SCFAs) profiles of normal and overweight school children in Selangor after probiotics administration. J. Funct. Foods 2009, 57, 103–111. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, depression, and the microbiome: A role for gut peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, B.; Gardner, H. The microbiome and cancer. J. Pathol. 2018, 244, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization (FAO). Guidelines for the Evaluation of Probiotics in Food; Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO: London, ON, Canada, 2002. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Goldin, B.R.; Gorbach, S.L. Effect of Lactobacillus acidophilus dietary supplements on 1,2-dimethylhydrazine dihydrochloride-induced intestinal cancer in rats. J. Natl. Cancer Inst. 1980, 64, 263–265. [Google Scholar] [CrossRef]
- Altonsy, M.O.; Andrews, S.C.; Tuohy, K.M. Differential induction of apoptosis in human colonic carcinoma cells (Caco-2) by Atopobium, and commensal, probiotic and enteropathogenic bacteria: Mediation by the mitochondrial pathway. Int. J. Food Microbiol. 2010, 137, 190–203. [Google Scholar] [CrossRef]
- Borowicki, A.; Michelmann, A.; Stein, K.; Scharlau, D.; Scheu, K. Fermented wheat aleurone enriched with probiotic strains LGG and Bb12 modulates markers of tumor progression in human colon cells. Nutr. Cancer. 2011, 63, 151–160. [Google Scholar] [CrossRef]
- Orlando, A.; Refolo, M.G.; Messa, C.; Amati, L.; Lavermicocca, P.; Guerra, V.; Russo, F. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr. Cancer 2012, 64, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Orlando, A.; Linsalata, M.; Cavallini, A.; Messa, C. Effects of Lactobacillus rhamnosus GG on the cell growth and polyamine metabolism in HGC-27 human gastric cancer cells. Nutr. Cancer. 2007, 59, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Aliabadi, H.; Mohammadi, F.; Fazeli, H.; Mirlohi, M. Effects of Lactobacillus plantarum A7 with probiotic potential on colon cancer and normal cells proliferation in comparison with a commercial strain. Iran. J. Basic. Med. Sci. 2014, 17, 815–819. [Google Scholar]
- Lopez, M.; Li, N.; Kataria, J.; Russell, M.; Neu, J. Live and ultraviolet-inactivated Lactobacillus rhamnosus GG decrease flagellin-induced interleukin-8 production in Caco-2 cells. J. Nutr. 2008, 138, 2264–2268. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, C.; Millette, M.; Oth, D.; Ruiz, M.T.; Luquet, F.M.; Lacroix, M. Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracilinduced apoptosis. Nutr. Cancer. 2010, 62, 371–378. [Google Scholar] [CrossRef]
- Lee, J.W.; Shin, J.G.; Kim, E.H.; Kang, H.E.; Yim, I.B.; Kim, J.Y.; Joo, H.G.; Woo, H.J. Immunomodulatory and antitumor effects in vivo by the cytoplasmic fraction of Lactobacillus casei and Bifidobacterium longum. J. Vet. Sci. 2004, 5, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.L.; Choi, Y.J.; Choi, J.; Pothoulakis, C.; Rhee, S.H.; Imet, E. The anticancer effect of probiotic Bacillus polyfermenticus on human colon cancer cells is mediated through ErbB2 and ErbB3 inhibition. Int. J. Cancer. 2010, 127, 780–790. [Google Scholar] [PubMed] [Green Version]
- Saxami, G.; Karapetsas, A.; Lamprianidou, E.; Kotsianidis, I.; Chlichlia, A.; Tassou, C.; Zoumpourliset, V.; Galanis, A. Two potential probiotic lactobacillus strains isolated from olive microbiota exhibit adhesion and anti-proliferative effects in cancer cell lines. J. Funct. Foods 2016, 24, 461–471. [Google Scholar] [CrossRef]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lampranidou, E.; Saxami, G.; Ypsilantis, P.; Lampri, E.; Simopoulos, C.; et al. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef]
- Han, K.J.; Lee, N.K.; Park, H.; Paik, H.D. Anticancer and anti-inflammatory activity of probiotic Lactococcus lactis nk34. J. Microbiol. Biotechnol. 2015, 25, 1697–1701. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.K.; Son, S.H.; Jeon, E.B.; Jung, G.H.; Lee, J.Y.; Paik, H.D. The prophylactic effect of probiotic Bacillus polyfermenticus KU3 against cancer cells. J. Funct. Foods 2015, 14, 513–518. [Google Scholar] [CrossRef]
- Chen, Z.F.; Ai, L.Y.; Wang, J.L.; Ren, L.L.; Yu, Y.N.; Xu, J.; Chen, H.Y.; Yu, J.; Li, M.; Qin, W.X.; et al. Probiotics Clostridium butyricum and Bacillus subtilis ameliorate intestinal tumorigenesis. Future. Microbiol. 2015, 10, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; Gimzewski, J. Apoptotic effect of a novel kefir product, PFT, on multidrug-resistant myeloid leukemia cells via a hole-piercing mechanism. Int. J. Oncol. 2014, 44, 830–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thirabunyanon, M.; Hongwittayakorn, P. Potential probiotic lactic acid bacteria of human origin induce antiproliferation of colon cancer cells via synergic actions in adhesion to cancer cells and short-chain fatty acid bioproduction. Appl. Biochem. Biotechnol. 2013, 169, 511–525. [Google Scholar] [CrossRef]
- Cousin, F.J.; Jouan-Lanhouet, S.; Dimanche-Boitrel, M.T.; Corcos, L.; Jan, G. Milk fermented by Propionibacterium freudenreichii induces apoptosis of HGT-1 human gastric cancer cells. PLoS ONE 2012, 7, 31892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Oh, S.; Yun, H.S.; Oh, S.; Kim, S.H. Cell-bound exopolysaccharide from probiotic bacteria induces autophagic cell death of tumour cells. Lett. Appl. Microbiol. 2010, 51, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.S.; Molina, M.A.; Di Sciullo, P.; Azpiroz, M.B.; Leocata Nieto, F.; Sterín-Speziale, N.B.; Mongini, C.; Manghi, M.A. Beneficial activity of Enterococcus faecalis CECT7121 in the anti-lymphoma protective response. J. Appl. Microbiol. 2010, 109, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Thirabunyanon, M.; Boonprasom, P.; Niamsup, P. Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnol. Lett. 2009, 31, 571–576. [Google Scholar] [CrossRef]
- Iyer, C.; Kosters, A.; Sethi, G.; Kunnumakkara, A.B.; Aggarwal, B.B.; Versalovic, J. Probiotic Lactobacillus reuteri promotes TNFinduced apoptosis in human myeloid leukemia-derived cells by modulation of NF-kappaB and MAPK signalling. Cell. Microbiol. 2008, 10, 1442–1452. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Kim, D.; Cho, J.; Yang, J.; Chung, M.; Kim, K.; Ha, N. Inhibition of proliferation in colon cancer cell lines and harmful enzyme activity of colon bacteria by Bifidobacterium adolescentis SPM0212. Arch. Pharm. Res. 2008, 31, 468–473. [Google Scholar] [CrossRef]
- Jan, G.; Belzacq, A.S.; Haouzi, D.; Rouault, A.; Metivier, D.; Kroemer, G.; Brenner, C. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 2002, 9, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Dubey, V.; Ghosh, A.R.; Bishayee, K.; Khuda-Bukhsh, A.R. Appraisal of the anti-cancer potential of probiotic Pediococcus pentosaceus GS4 against colon cancer: In vitro and in vivo approaches. J. Funct. Foods 2016, 23, 66–79. [Google Scholar] [CrossRef]
- Talero, E.; Bolivar, S.; Ávila-Román, J.; Alcaide, A.; Fiorucci, S.; Motilva, V. Inhibition of chronic ulcerative colitis-associated adenocarcinoma development in mice by VSL#3. Inflamm. Bowel. Dis. 2015, 21, 1027–1037. [Google Scholar] [PubMed] [Green Version]
- Lenoir, M.; del Carmen, S.; Cortes-Perez, N.G.; Lozano-Ojalvo, D.; Muñoz-Provencio, D.; Chain, F.; Langella, P.; de Moreno de LeBlanc, A.; LeBlanc, J.G.; Bermúdez-Humarán, L.G. Lactobacillus casei BL23 regulates Tregand Th17 T-cell populations and reduces DMH-associated colorectal cancer. J. Gastroenterol. 2016, 51, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Gamallat, Y.; Meyiah, A.; Kuugbee, E.D.; Chiwala, G.; Awadasseid, A.; Bamba, D.; Zhang, X.; Shang, X.; Luo, F.; Xin, Y. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomed. Pharmacother. 2016, 83, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Kuugbee, E.D.; Shang, X.; Gamallat, Y.; Bamba, D.; Awadasseid, A.; Suliman, M.A.; Zang, S.; Ma, Y.; Chiwala, G.; Xin, Y.; et al. Structural change in microbiota by a probiotic cocktail enhances the gut barier and reduces cancer via TLR2 signaling in a rat model of colon cancer. Dig. Dis. Sci. 2016, 61, 2908–2920. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, X.; Fang, B.; Zhu, C.; Zhu, J.; Ren, F. Effects of Lactobacillus salivarius Ren on cancer prevention and intestinal microbiota in 1, 2-dimethylhydrazine-induced rat model. J. Microbiol. 2015, 53, 398–405. [Google Scholar] [CrossRef]
- Walia, S.; Kamal, R.; Dhawan, D.K.; Kanwar, S.S. Chemoprevention by probiotics during 1,2-dimethylhydrazine-induced colon carcinogenesis in rats. Dig. Dis. Sci. 2018, 63, 900–909. [Google Scholar] [CrossRef]
- Hu, J.; Wang, C.; Ye, L.; Yang, W.; Huang, H.; Meng, F.; Shi, S.; Ding, Z. Anti-tumour immune effect of oral administration of Lactobacillus plantarum to CT26 tumourbearing mice. J. Biosci. 2015, 40, 269–279. [Google Scholar] [CrossRef]
- Verma, A.; Shukla, G. Synbiotic (Lactobacillus rhamnosus + Lactobacillus acidophilus + inulin) attenuates oxidative stress and colonic damage in 1,2 dimethylhydrazine dihydrochloride-induced colon carcinogenesis in Sprague’ Dawley rats: A long-term study. Eur. J. Cancer Prev. 2014, 23, 550–559. [Google Scholar] [CrossRef]
- Chang, J.H.; Shim, Y.Y.; Cha, S.K.; Reaney, M.J.T.; Chee, K.M. Effect of Lactobacillus acidophilus KFRI342 on the development of chemically induced precancerous growths in the rat colon. J. Med. Microbiol. 2012, 61, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosai, V.; Ambalam, P.; Raman, M.; Kothari, C.R.; Kothari, R.K.; Vyas, B.R.M.; Sheth, N.R. Protective effect of Lactobacillus rhamnosus 231 against N-Methyl-N’-nitro-N-nitrosoguanidine in animal model. Gut Microbes 2011, 2, 319–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appleyard, C.B.; Cruz, M.L.; Isidro, A.A.; Arthur, J.C.; Jobin, C.; De Simone, C. Pretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am. J. Physiol. Liver Physiol. 2011, 301, G1004–G1013. [Google Scholar]
- Bertkova, I.; Hijova, E.; Chmelarova, A.; Mojzisova, G.; Petrasova, D.; Strojny, L.; Bomba, A.; Zitnan, R. The effect of probiotic microorganisms and bioactive compounds on chemically induced carcinogenesis in rats. Neoplasma 2010, 57, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Singh, N.K.; Sinha, P.R. Inhibition of 1,2-dimethylhydrazine induced colon genotoxicity in rats by the administration of probiotic curd. Mol. Biol. Rep. 2010, 37, 1373–1376. [Google Scholar] [CrossRef]
- Park, E.; Jeon, G.I.; Park, J.S.; Paik, H.D. A probiotic strain of Bacillus polyfermenticus reduces DMH induced precancerous lesions in F344 male. Rat. Biol. Pharm. Bull. 2007, 30, 569–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafter, J.; Bennett, M.; Caderni, G.; Clune, Y.; Hughes, R.; Karlsson, P.; Klinder, A.; O’Riordan, M.; O’Sullivan, G.; Pool-Zobel, B.; et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007, 85, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Zhang, X.; Covasa, M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J. Gastroenterol. 2014, 20, 7878–7886. [Google Scholar] [CrossRef] [PubMed]
- Ohigashi, S.; Hoshino, Y.; Ohde, S.; Onodera, H. Functional outcome, quality of life, and efficacy of probiotics in postoperative patients with colorectal cancer. Surg. Today 2011, 41, 1200–1206. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, H.; Yang, Z.; Xia, Y.; Liu, W.; Yang, J.; Jiang, Y.; Zhang, H.; Wang, Y.; Zheng, Q. Randomized clinical trial: The effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery-A double-blind study. Aliment. Pharmacol. Ther. 2011, 33, 50–63. [Google Scholar] [CrossRef]
- Österlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Nezami, H.S.; Polychronaki, N.N.; Ma, J.; Zhu, H.; Ling, W.; Salminen, E.K.; Juvonen, R.O.; Salminen, S.J.; Poussa, T.; Mykkänen, H.M. Probiotic supplementation reduces a biomarker for increased risk of liver cancer in young men from Southern China. Am. J. Clin. Nutr. 2006, 83, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, B.S.; Ghadimi-Darsajini, A.; Nekouian, R.; Iragian, G.R. In vitro activity of probiotic Lactobacillus reuteri against gastric cancer progression by downregulation of urokinase plasminogen activator/urokinase plasminogen activator receptor gene expression. J. Cancer Res. Ther. 2017, 13, 246–251. [Google Scholar] [PubMed]
- Ghoneum, M.; Felo, N. Selective induction of apoptosis in human gastric cancer cells by Lactobacillus kefiri (PFT), a novel kefir product. Oncol. Rep. 2015, 34, 1659–1666. [Google Scholar] [CrossRef] [Green Version]
- Chitapanarux, I.; Chitapanarux, T.; Traisathit, P.; Kudumpee, S.; Tharavichitkul, E.; Lorvidhaya, V. Randomized controlled trial of live Lactobacillus acidophilus plus Bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat. Oncol. 2010, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Toi, M.; Hirota, S.; Tomotaki, A. Probiotic beverage with soy isoflavone consumption for breast cancer prevention: A case-control study. Curr. Nutr. Food Sci. 2013, 9, 194–200. [Google Scholar] [CrossRef]
- Pala, V.; Sieri, S.; Berrino, F.; Vineis, P.; Sacerdote, C.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2011, 129, 2712–2719. [Google Scholar] [CrossRef]
- Ohara, T.; Yoshino, K.; Kitajima, M. Possibility of preventing colorectal carcinogenesis with probiotics. Hepatogastroenterol 2010, 57, 1411–1415. [Google Scholar]
- Hatakka, K.; Holma, R.; El-Nezami, H.; Suomalainen, T.; Kuisma, M.; Saxelin, M.; Poussa, T.; Mykkänen, H.; Korpela, R. The influence of Lactobacillus rhamnosus LC705 together with Propionibacterium freudenreichii ssp. shermanii JS on potentially carcinogenic bacterial activity in human colon. Int. J. Food Microbiol. 2008, 128, 406–410. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakai, S.; Tsukamoto, T. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol. Int. 2002, 68, 273–280. [Google Scholar] [CrossRef]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A four-probiotics regimen reduces postoperative complications after colorectal surgery: A randomized, double-blind, placebo-controlled study. World J. Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Huang, M.J.; Zhang, X.W.; Wang, L.; Huang, N.Q.; Peng, H.; Lan, P.; Peng, J.S.; Yang, Z.; Xia, Y.; et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: A double-center and doubleblind randomized clinical trial. Am. J. Clin. Nutr. 2013, 97, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.W.; Peng, D.; Yang, B.R.; Gao, J.; Fang, W.J.; Ying, C.M. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am. J. M. Sci. 2012, 343, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Nagata, S.; Saito, M.; Shimizu, T.; Yamashiro, Y.; Matsuki, T.; Asahara, T.; Nomoto, K. Effects of the enteral administration of Bifidobacterium breve on patients undergoing chemotherapy for pediatric malignancies. Support. Care Cancer 2010, 18, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, S.A.; Da Conceição, L.L.; Siqueira, N.P.; Rosa, D.D.; Da Silva, L.L.; Peluzio, M.C.G. Review of the mechanisms of probiotic actions in the prevention of colorectal cancer. Nutr. Res. 2017, 37, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Koboziev, I.; Webb, C.R.; Furr, K.L.; Grisham, M.B. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free. Radic. Biol. Med. 2013, 68, 122–133. [Google Scholar] [CrossRef]
- Sobhani, I.; Tap, J.; Roudot-Thoraval, F.; Roperch, J.P.; Letulle, S.; Langella, P.; Corthier, G.; Tran Van Nhieu, J.; Furet, J.P. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE 2011, 6, e16393. [Google Scholar] [CrossRef]
- Kahouli, I.; Tomaro-Duchesneau, C.; Prakash, S. Probiotics in colorectal cancer (CRC) with emphasis on mechanisms of action and current perspectives. J. Med. Microbiol. 2013, 62, 1107–1123. [Google Scholar] [CrossRef] [Green Version]
- Boleij, A.; Hechenbleikner, E.M.; Goodwin, A.C.; Badani, R.; Stein, E.M.; Lazarev, M.G.; Ellis, B.; Carroll, K.C.; Albesiano, E.; Wick, E.C. The bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 2015, 60, 208–215. [Google Scholar] [CrossRef]
- Ambalam, P.; Raman, M.; Purama, R.K.; Doble, M. Probiotics, prebiotics and colorectal cancer prevention. Best. Pract. Res. Clin. Gastroenterol. 2016, 30, 119–131. [Google Scholar] [CrossRef]
- Molska, M.; Reguła, J. Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients 2019, 11, 2453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javanmard, A.; Ashtari, S.; Sabet, B.; Davoodi, S.H.; Rostami-Nejad, M.; Akbari, M.E.; Niaz, A.; Mortazavian, A.M. Probiotics and their role in gastrointestinal cancers prevention and treatment; an overview. Gastroenterol. Hepatol. 2018, 11, 284–295. [Google Scholar]
- Fooks, L.; Gibson, G. Probiotics as modulators of the gut flora. Br. J. Nutr. 2002, 88, S39–S49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohland, C.L.; MacNaughton, W.K. Probiotic bacteria and intestinal barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, 807–819. [Google Scholar] [CrossRef] [Green Version]
- Davey, M.E.; O’Toole, G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef] [Green Version]
- Dieltjens, L.; Appermans, K.; Lissens, M.; Lories, B.; Kim, W.; Van der Eycken, E.V.; Foster, K.R.; Steenackers, H.P. Inhibiting bacterial cooperation is an evolutionarily robust anti-biofilm strategy. Nat. Commun. 2020, 107, 1–11. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, R.; Wu, W.; Qin, H. The role of gut microbiota in the pathogenesis of colorectal cancer. Tumor Biol. 2013, 34, 1285–1300. [Google Scholar] [CrossRef]
- Goldin, B.R.; Gorbach, S.L. The effect of milk and Lactobacillus feeding on human intestinal bacterial enzyme activity. Am. J. Clin. Nutr. 1984, 39, 756–761. [Google Scholar] [CrossRef]
- Tjalsma, H.; Boleij, A.; Marchesi, J.R.; Dutilh, B.E. A bacterial driverpassenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef]
- Rountree, R. Proven therapeutic benefits of high-quality probiotics. Appl. Nutr. Sci. Rep. 2002, 4, 1–6. [Google Scholar]
- Boris, S.; Barbés, C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes. Infect. 2000, 2, 543–546. [Google Scholar] [CrossRef]
- Eklund, T. Organic acids and esters. In Mechanism of Actions of Food Preservation Procedures; Gould, G.W., Ed.; Scientific Publishers: London, UK, 1989; pp. 161–200. [Google Scholar]
- Fung, W.Y.; Lye, H.S.; Lim, T.J.; Kuan, C.Y.; Liong, M.T. Role of probiotic on gut health. In Probiotics. Biology, genetics and Health Aspects; Liong, M.T., Ed.; Springer: New York, NY, USA, 2001; pp. 139–166. [Google Scholar]
- Dembele, T.; Obdrzalek, V.; Votava, M. Inhibition of bacterial pathogens by lactobacilli. Zentralbl. Bakteriol. 1998, 288, 395–401. [Google Scholar] [CrossRef]
- Tomás, M.S.; Otero, C.M.; Ocaña, V.; Nader-Macias, E.M. Production of antimicrobial substances by lactic acid bacteria I: Determination of hydrogen peroxide. Meth. Mol. Biol. 2004, 268, 337–346. [Google Scholar]
- Klaenhammer, T.R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 39–86. [Google Scholar] [CrossRef]
- Nazir, Y.; Hussain, S.A.; Hamid, A.A.; Song, Y. Probiotics and their potential preventive and therapeutic role for cancer, high serum cholesterol, and allergic and HIV diseases. BioMed Res. Int. 2018, 2018, 1–17. [Google Scholar] [CrossRef]
- Lievin, V.; Peifer, I.; Hudault, S. Bifdobacterium strains from resident infant human gastrointestinal microfora exert antimicrobial activity. Gut 2000, 47, 646–652. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, D.S.; Cho, G.S.; Hanak, A.; Huch, M.; Franz, C.M.; Arneborg, N. The effect of bacteriocin-producing Lactobacillus plantarum strains on the intracellular pH of sessile and planktonic Listeria monocytogenes single cells. Int. J. Food Microbiol. 2010, 141, S53–S59. [Google Scholar] [CrossRef]
- Drissi, F.; Buffet, S.; Raoult, D.; Merhej, V. Common occurrence of antibacterial agents in human intestinal microbiota. Front. Microbiol. 2015, 6, 441. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Roediger, W.E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 1980, 21, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Kuczyńska, B.; Wasilewska, A.; Biczysko, M.; Banasiewicz, T.; Drews, M. Krótkołańcuchowe kwasy tłuszczowe—Mechanizm działania, potencjalne zastosowanie kliniczne oraz zalecenia dietetyczne. Now. Lek. 2011, 80, 299–304. (In Polish) [Google Scholar]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Sharma, R.; Frost, G. Propionate. Anti-obesity and satiety factor? Appetite 2011, 56, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Hautefort, I.; Thompson, A.; Hinton, J.C.; Van Immerseel, F. Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Appl. Environ. Microbiol. 2006, 72, 946–949. [Google Scholar] [CrossRef] [Green Version]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Kumar, M.; Nagpal, R.; Verma, V.; Kumar, A.; Kaur, N.; Hemalatha, R. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr. Rev. 2012, 71, 23–34. [Google Scholar] [CrossRef]
- Duc, N.M.; Kim, H.R.; Chung, K.Y. Structural mechanism of G protein activation by G protein-coupled receptor. Eur. J. Pharm. 2015, 763, 214–222. [Google Scholar] [CrossRef]
- Soel, S.M.; Choi, O.S.; Bang, M.H.; Yoon Park, J.H.; Kim, W.K. Influence of conjugated linoleic acid isomers on the metastasis of colon cancer cells in vitro and in vivo. J. Nutr. Biochem. 2007, 18, 650–657. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Viladomiu, M.; Pedragosa, M.; Simone, C.; Hontecillas, R. Immunoregulatory mechanisms underlying prevention of colitis-associated colorectal cancer by probiotic bacteria. PLoS ONE 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Walker, J.W.; Diaz, H.; Madsen, K.L. Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J. Nutr. 2006, 136, 1483–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fearon, E.R. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. Mech. 2011, 6, 479–507. [Google Scholar] [CrossRef] [PubMed]
- De Vries, E.G.; Gietema, J.A.; de Jong, S. Tumor necrosis factor-related apoptosis-inducing ligand pathway and its therapeutic implications. Clin. Cancer Res. 2006, 12, 2390–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.W.; Baek, Y.M.; Yang, K.E.; Yoo, H.S.; Cho, C.K.; Lee, Y.W. Lactobacillus casei extract induces apoptosis in gastric cancer by inhibiting NF-κB and mTOR-mediated signalling. Integr. Cancer Ther. 2013, 12, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Yu, Y.N.; Wang, J.L.; Lin, Y.W.; Kong, X.; Yang, C.Q. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am. J. Clin. Nutr. 2013, 97, 1044–1052. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.T.; Chu, F.J.; Chou, C.C.; Yu, R.C. Antiproliferative and anticytotoxic effects of cell fractions and exopolysaccharides from Lactobacillus casei 01. Mutat. Res. 2011, 721, 157–162. [Google Scholar] [CrossRef]
- Nami, Y.; Abdullah, N.; Haghshenas, B.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Probiotic potential and biotherapeutic effects of newly isolated vaginal Lactobacillus acidophilus 36YL strain on cancer cells. Anaerobe 2014, 28, 29–36. [Google Scholar] [CrossRef]
- Shinnoh, M.; Horinaka, M.; Yasuda, T.; Yoshikawa, S.; Morita, M. Clostridium butyricum MIYAIRI 588 shows antitumor effects by enhancing the release of TRAIL from neutrophils through MMP-8. Int. J. Oncol. 2013, 42, 903–911. [Google Scholar] [CrossRef] [Green Version]
- Klusek, J.; Głuszek, S.; Klusek, J. GST gene polymorphisms and the risk of colorectal cancer development. Contemp. Oncol. (Pozn.) 2014, 18, 219–221. [Google Scholar] [CrossRef] [Green Version]
- Pool-Zobel, B.; Veeriah, S.; Bohmer, F.D. Modulation of xenobiotic metabolising enzymes by anticarcinogens—Focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat. Res. 2005, 591, 74–92. [Google Scholar] [CrossRef]
- McIntosh, F.M.; Maison, N.; Holtrop, G.; Young, P.; Stevens, V.J.; Ince, J.; Johnstone, A.M.; Lobley, G.E.; Flint, H.J.; Louis, P. Phylogenetic distribution of genes encoding b-glucuronidase activity in human colonic bacteria and the impact of diet on faecal glycosidase activities. Environ. Microbiol. 2013, 14, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; De Preter, V.; Windey, K.; Verbeke, K. Functional analysis of colonic bacterial metabolism: Relevant to health? Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1–G9. [Google Scholar] [CrossRef] [PubMed]
- Culpepper, B.S.T.; Mai, V. Evidence for contributions of gut microbiota to colorectal carcinogenesis. Curr. Nutr. Rep. 2013, 2, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Commane, D.; Hughes, R.; Shortt, C.; Rowland, I. The potential mechanisms involved in the anti-carcinogenic action of probiotics. Mutat. Res. 2005, 591, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Shukla, G. Probiotics Lactobacillus rhamnosus GG, Lactobacillus acidophilus suppresses DMH-induced procarcinogenic fecal enzymes and preneoplastic aberrant crypt foci in early colon carcinogenesis in sprague dawley rats. Nutr. Cancer 2013, 65, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Jang, S.; Baek, E.H.; Kim, M.J.; Lee, K.S.; Shin, H.S.; Chung, M.J.; Kim, J.E.; Lee, K.O.; Ha, N.J. Lactic acid bacteria affect serum cholesterol levels, harmful fecal enzyme activity, and fecal water content. Lipids Health Dis. 2009, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bouhnik, Y.; Flourie, B.; Andrieux, C.; Bisetti, N.; Briet, F.; Rambaud, J. Effects of Bifidobacterium sp fermented milk ingested with or without inulin on colonic bifidobacteria and enzymatic activities in healthy humans. Eur. J. Clin. Nutr. 1996, 50, 269–273. [Google Scholar]
- Ling, W.; Korpela, R.; Mykkänen, H.; Salminen, S.; Hänninen, O. Lactobacillus strain GG supplementation decreases colonic hydrolytic and reductive enzyme activities in healthy female adults. J. Nutr. 1994, 124, 18–23. [Google Scholar] [CrossRef]
- Burns, A.J.; Rowland, I.R. Antigenotoxicity of probiotics and prebiotics on faecal water-induced DNA damage in human colon adenocarcinoma cells. Mutat. Res. 2004, 551, 233–243. [Google Scholar] [CrossRef]
- Bolognani, F.; Rumney, C.J.; Rowland, I.R. Influence of carcinogen binding by lactic acid-producing bacteria on tissue distribution and in vivo mutagenicity of dietary carcinogens. Food Chem. Toxicol. 1997, 35, 535–545. [Google Scholar] [CrossRef]
- Orrhage, K.; Sillerstrom, E.; Gustafsson, J.A.; Nord, C.E.; Rafter, J. Binding of mutagenic heterocyclic amines by intestinal and lactic acid bacteria. Mutat. Res. 1994, 311, 239–248. [Google Scholar] [CrossRef]
- Rowland, I.R.; Grasso, P. Degradation of N-nitrosamines by intestinal bacteria. Appl. Envir. Microbiol. 1975, 29, 7–12. [Google Scholar] [CrossRef]
- Morotomi, M.; Mutai, M. In vitro binding of potent mutagenic pyrolysates to intestinal bacteria. J. Nat. Cancer Instit. 1986, 11, 195–201. [Google Scholar]
- El-Nezami, H.; Kankaanp, P.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998, 36, 321–332. [Google Scholar] [CrossRef]
- Praveena, Y.S.N.; Padmini, P.C. Antibacterial activities of mycotoxins from newly isolated filamentous fungi. Int. J. Plant. Anim. Enviromental Sci. 2011, 1, 8–13. [Google Scholar]
- Gill, H.S.; Cross, M.L. Probiotics and immune function. In Nutrition and Immune Function; Calder, P.C., Field, C.J., Gill, H.S., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 251–272. [Google Scholar]
- Borchers, A.T.; Selmi, C.; Meyers, F.J.; Keen, C.L.; Gershwin, M.E. Probiotics and immunity. J. Gastroenterol. 2009, 44, 26–46. [Google Scholar] [CrossRef] [Green Version]
- Gόmez-Llorente, C.; Munoz, S.; Gil, A. Role of toll-like receptors in the development of immunotolerance mediated by probiotics. Proc. Nutr. Soc. 2010, 69, 381–389. [Google Scholar] [CrossRef]
- Urbanska, A.M.; Paul, A.; Bhahena, J.; Prakash, S. Suppression of tumorigenesis: Modulation of inflammatory cytokines by oral administration of microencapsulated probiotic yogurt formulation. Int. J. Inflam. 2010, 31, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, T.; Asai, Y.; Tamai, R.; Makimura, Y.; Sakamoto, H.; Hashikawa, S.; Yasuda, K. Natural killer cell activities of synbiotic Lactobacillus casei ssp. casei in conjunction with dextran. Clin. Exp. Immunol. 2006, 143, 103–109. [Google Scholar] [CrossRef]
- Sobhani, I.; Tap, J.; Roudot-Thoraval, F.; Roperch, J.P.; Letulle, S.; Langella, P.; Takeda, K.; Suzuki, T.; Shimada, S.I.; Shida, K.; et al. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei shirota. Clin. Exp. Immunol. 2006, 146, 109–115. [Google Scholar]
- Madsen, K.L. Enhancement of epithelial barrier function by probiotics. J. Epithel. Biol. Pharmacol. 2012, 5, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Ohigashi, S.; Sudo, K.; Kobayashi, D.; Takahashi, O.; Takahashi, T.; Asahara, T. Changes of the intestinal microbiota, short chain fatty acids, and fecal pH in patients with colorectal cancer. Dig. Dis. Sci. 2013, 58, 1717–1726. [Google Scholar] [CrossRef]
- Crawford, N.; Brooke, B.N. The pH and buffering power of human bile. Lancet 1955, 268, 1096–1097. [Google Scholar] [CrossRef]
- Shimizu, K.; Ogura, H.; Goto, M.; Asahara, T.; Nomoto, K.; Morotomi, M.; Yoshiya, K.; Matsushima, A.; Sumi, Y.; Kuwagata, Y. Altered gut flora and environment in patients with severe SIRS. J. Trauma 2006, 60, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Yajima, T. Correlation between water-holding capacity of different types of cellulose in vitro and gastrointestinal retention time in vivo of rats. J. Sci. Food Agric. 1992, 60, 139–146. [Google Scholar] [CrossRef]
- Caballero-Franco, C.; Keller, K.; De Simone, C.; Chadee, K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, 315–322. [Google Scholar]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 289, 851–859. [Google Scholar] [CrossRef] [Green Version]



| Reference | Probiotic Strain | Cell Line | Effect |
|---|---|---|---|
| [20] | Lactobacillus pentosus B281 Lactobacillus plantarum B282 | Caco-2 and HT-29 | ↓ Cell proliferation, Cell cycle arrest (G1) |
| [21] | Lactobacillus casei ATCC 393 | HT29 and CT26 | Induction of apoptosis |
| [22] | Lactococcus lactis NK34 | HT-29, LoVo, AGS | >80% ↓ Cell proliferation |
| [23] | Bacillus polyfermenticus KU3 | LoVo, HT-29, AGS | >90% ↓ Cell proliferation |
| [24] | Clostridium butyricum ATCC Bacillus subtilis ATCC 9398 | HCT116, SW1116, Caco-2 | ↓ Cell proliferation |
| [15] | Lactobacillus plantarum A7 Lactobacillus rhamnosus GG | Caco-2, HT-29 | ↓ Cell proliferation |
| [25] | Lactobacillus kefiri P-IF | MDR | Induction of apoptosis |
| [26] | Pediococcus pentosaceus FP3 Lactobacillus salivarius FP25/FP35 Enterococcus faecium FP51 | Caco-2 | ↓ Cell proliferation, Activation of apoptosis |
| [27] | Propionibacterium freudenreichii ITG P9 | HGT-1 | Induction of apoptosis |
| [13] | Lactobacillus paracasei IMPC2.1 Lactobacillus rhamnosus GG | DLD-1, HGC-27 | ↓ Cell proliferation, Induction of apoptosis |
| [12] | Lactobacillus rhamnosus GG Bifidobacterium lactis Bb12 | HT-29 | Induction of apoptosis |
| [17] | Lactobacillus acidophilus CL1285 Lactobacillus casei LBC80R (in the presence of 5-FU) | LS513 | 40% ↑ apoptosis |
| [28] | Lactobacillus acidophilus 606 | HT-29 | Inhibited proliferation of tumor cells |
| [19] | Bacillus polyfermenticus | NMC460 | ↓ Cell colony formation in cancer cells (N/E on normal colonocytes) |
| [29] | Enterococcus faecalis CECT7121 | LBC | Inhibited proliferation of tumor cells, Induction od apoptosis |
| [11] | Lactobacillus rhamnosus GG Bifidobacterium lactis Bb12 | Caco-2 | ↑ Apoptosis |
| [30] | Enterococcus faecium RM11 Lactobacillus fermentum RM28 | Caco-2 | Cell proliferation: ↓ 21% ↓ 23% |
| [31] | Lactobacillus reuteri ATCC PTA 6475 | KBM-5 | ↑ Apoptosis |
| [16] | Lactobacillus rhamnosus GG | Caco-2 | ↓ level of IL–8 |
| [32] | Bifidobacterium adolescentis SPM0212 | Caco-2, HT-29, SW480 | ↓ Cell proliferation |
| [14] | Lactobacillus rhamnosus GG | HGC-27 | ↓ Cell proliferation Induction of apoptosis |
| [18] | Lactobacillus acidophilus SNUL Lactobacillus casei YIT9029 Bifidobacterium longum HY8001 | SNUC2A, SNU1, NIH/3T3 and Jurkat cell | Suppressed proliferation of tumor cells |
| [33] | Propionibacterium acidopropionici CNRZ80 | HT-29 | ↓ Cell proliferation Induction of apoptosis |
| Reference | Probiotic Strain | Model | Induction | Time of Treatment | Effect |
|---|---|---|---|---|---|
| [36] | Lactobacillus casei BL23 | C57BL/6 mice | DMH | 10 weeks | ↓ TI |
| [34] | Pediococcus pentosaceus GS4 | Swiss albino mice | AOM | 4 weeks | ↓ TP Induction of apoptosis |
| [37] | Lactobacillus rhamnosus GG CGMCC 1.2134 | SD rats | DMH 10 weeks | 25 weeks | ↓ TI ↓ TV ↓ TM Induction of apoptosis |
| [38] | Lactobacillus acidophilus Bifidobacterium bifidum Bifidobacterium infantum | SD rats | antibiotics DMH | 23 weeks | ↓ TI ↓ TV |
| [39] | Lactobacillus salivarius Ren | F344 rats | DMH 10 weeks | 2 weeks a | ↓ TI |
| [40] | Lactobacillus plantarum (AdF10) Lactobacillus rhamnosus GG | SD rats | DMH 4 weeks | One of strains 12 weeks | ↓ TI ↓ TV ↓ TM |
| [15] | VSL#3 (Probiotics mixture) | C57BL/6 mice | DSS | 2 weeks a | ↓ TI ↓ dysplasia |
| [41] | Lactobacillus plantarum | BALB/c mice | CT26 cells injection | 14 weeks | ↓ TV Induction of necrosis |
| [23] | Lactobacillus plantarum | BALB/c mice | AOM, DSS | Nanosized/ Live bacteria 4 weeks | ↓ TI cell cycle arrest Induction of apoptosis |
| [42] | Lactobacillus rhamnosus GG MTCC #1408 Lactobacillus acidophilus NCDC #1 | SD rats | DMH | 19 weeks a | ↓ TI ↓ TM |
| [43] | Lactobacillus acidophilus KFRI342 | F344 rats | DMH | 10 weeks | ↓ ACF ↓ β-glucuronidase and β-glucosidase activity |
| [44] | Lactobacillus rhamnosus 231 (Lr 231) | rats | MNNG | 5 weeks | ↓ fecal activity of azoreductase and nitroreductase, ↓ GST ↑GSH |
| [45] | VSL#3 (Probiotics mixture) | SD rats | TNBS | 10 weeks | None of the animals developed CRC |
| [46] | Lactobacillus plantarum | Wistar albino rats | DMH | 6 weeks | ↓ the activities of bacterial enzymes, the fecal bile acids concentration ↑ serum TNFα level |
| [19] | Bacillus polyfermenticus | CD-1 mice | DLD-1 cells injection | 20 weeks (injection) | ↓ TI ↓ TV |
| [28] | Bifidobacterium lactis KCTC 5727 | SPF C57BL rat | – | 19 weeks | ↓ TI ↓ TV |
| [47] | Lactobacillus acidophilus, Lactobacillus casei Lactobacillus lactis biovar diacetylactis DRC-1 | Rat | DMH | 40 weeks | ↓ TI ↓TV ↓ TM |
| [48] | Bacillus polyfermenticus | F344 rats | DMH | 6 weeks | 50% ↓ ACF, ↑ antioxidant potential |
| Reference | Type of Cancer | Strain/Strains |
|---|---|---|
| [49] | Colorectal carcinoma | Bifidobacterium lactis Bb12, Lactobacillus rhamnosus GG |
| [50] | Bifidobacterium longum, Lactobacillus acidophilus, Enterococcus faecalis | |
| [51] | Bacillus natto, Lactobacillus acidophilus | |
| [52] | Lactobacillus plantarum CGMMCC No 1258, Lactobacillus acidophilus LA-11, Bifidobacterium longum BL-88 | |
| [53] | Lactobacillus rhamnosus 573 | |
| [54] | Liver cancer | Lactobacillus rhamnosus LC705, Propionibacterium freudenreichii subsp. shermanii |
| [55] | Gastric cancer | Lactobacillus reuteri PTCC 1655 |
| [56] | Lactobacillus kefiri P-IF | |
| [57] | Cervical cancer | Lactobacillus acidophilus, Bifidobacterium bifidum |
| Reference | Probiotic Strain | Subject | Time of Treatment | Effect |
|---|---|---|---|---|
| The Prevention | ||||
| [58] | Yakult containing Lactobacillus casei Shirota (LcS) and isoflavones from soy product | 968 breast cancer patients aged 40 to 55. | 2 years | ↓ the incidence of breast cancer in Japanese women correlated with consumption of LcS and isoflavones since adolescence. |
| [59] | Streptococcus thermophilus and Lactobacillus delbruckii subsp. bulgaricus | 45,241 healthy people (14,178 men, 31,063 women) | 12 years | ↓ the risk of colorectal cancer correlated with increased consumption of yogurt (especially in men). |
| [60] | Lactobacillus gasseri OLL2716 (LG21) | 10 people with colorectal cancer and 20 healthy patients | 12 weeks | the number of bacteria from the genus Lactobacillus, synthesis of isobutyric acid, NK cell activity. ↓ the amount of Clostridium perfringens. |
| [19] | Lactobacillus casei Shirota (LcS) | 54 women with an HPV-positive intra epithelial lesion | 6 months | 60 % ↓ in human papilloma virus (HPV) associated infection and cervical cancer precursors |
| [61] | Lactobacillus rhamnosus LC705, Propionibacterium freudenreichii ssp. shermanii JS | 38 men (between 24 and 55 years old) | 4 weeks | ↓ β-glucosidase and urease activity amount of bacteria of the genus Lactobacillus and Propionibacterium |
| [54] | Lactobacillus rhamnosus LC705, Propionibacterium freudenreichii subsp. shermanii | 90 male students with high aflatoxin level in urine | 5 weeks | 61.5% ↓ a liver cancer biomarker which leads to reduced urinary excretion of aflatoxin B1-N7guanine (AFB-N7-guanine) |
| [62] | Lactobacillus acidophilus L1 | 180 people with bladder cancer (mean age:67 years and 445 population-based controls | 10 weeks | ↓ the risk of bladder cancer correlated with habitual intake of lactic acid bacteria |
| The Treatment | ||||
| [63] | Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterium lactis, Saccharomyces boulardii | 164 patients with colorectal cancer undergoing colorectal surgery | 30 days | ↓ the risk of postoperative complications. In the probiotic group, a positive correlation was observed between the expression of the SOCS3 gene and the expression of the TNF gene and circulating IL–6. |
| [64] | Lactobacillus plantarum CGMCC, Lactobacillus acidophilus-11, Bifidobacterium longum-88 | 150 patients with colorectal cancer | 6 days preoperatively and 10 days postoperative | ↓ the serum zonulin concentration, ↓ duration of postoperative pyrexia, ↓ duration of antibiotic therapy, ↓ rate of postoperative infectious complications, Inhibited the p38 mitogen-activated protein kinase signaling pathway |
| [65] | Bifidobacterium longum | 60 patients with colorectal cancer undergoing colon resection | 3 days | the count of bacteria of the genus Bifidobacterium ↓ the count of bacteria of the genus Escherichia |
| [66] | Bifidobacterium breve Yakult | 42 patients during chemotherapy | 6 weeks | ↓ the incidence of fever ↓ the need for intravenous antibiotics using compared to the control group. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The Role of Probiotics in Cancer Prevention. Cancers 2021, 13, 20. https://doi.org/10.3390/cancers13010020
Śliżewska K, Markowiak-Kopeć P, Śliżewska W. The Role of Probiotics in Cancer Prevention. Cancers. 2021; 13(1):20. https://doi.org/10.3390/cancers13010020
Chicago/Turabian StyleŚliżewska, Katarzyna, Paulina Markowiak-Kopeć, and Weronika Śliżewska. 2021. "The Role of Probiotics in Cancer Prevention" Cancers 13, no. 1: 20. https://doi.org/10.3390/cancers13010020